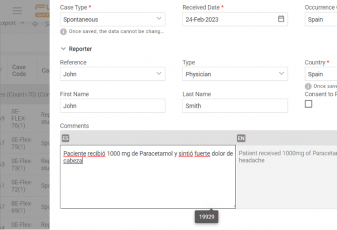
With Flex Databases Pharmacovigilance, you’ll only need one case, and you can add as many languages to it as you want.
The development of information technology is significantly ahead of the development of regulatory and other legislation. Despite the obvious benefits of introducing new technologies in electronic systems for pharmacovigilance, it is necessary to consider the difficulties that a company will face:
Validation and audits completion
Personal data management
Personnel training, possible changes in the organizational structure of the company
When we developed the Pharmacovigilance module, we considered all possible difficulties and propose the following solutions:
The entire pharmacovigilance process, from receiving the message to reporting and identifying safety signals, can be automated, thereby limiting the amount of human intervention that will still be required for exception handling, quality assurance, and analysis. Usage of automation is costly, but it will reduce data-processing costs, eliminate the potential for human error, and improve data quality and accuracy.
If you are interested in pharmacovigilance automation and want to learn more about the Flex Databases system – write to us at bd@flexdatabases.com or send a request for a demo through the form in the header of the page.
We are here for all of your questions! Tell us more about yourself and we will organize a tailored live demo to show how you can power up your clinical trials processes with Flex Databases.