Achieving compliance, streamlined training, and transparency in SOPs management with LMS
October 27, 2021
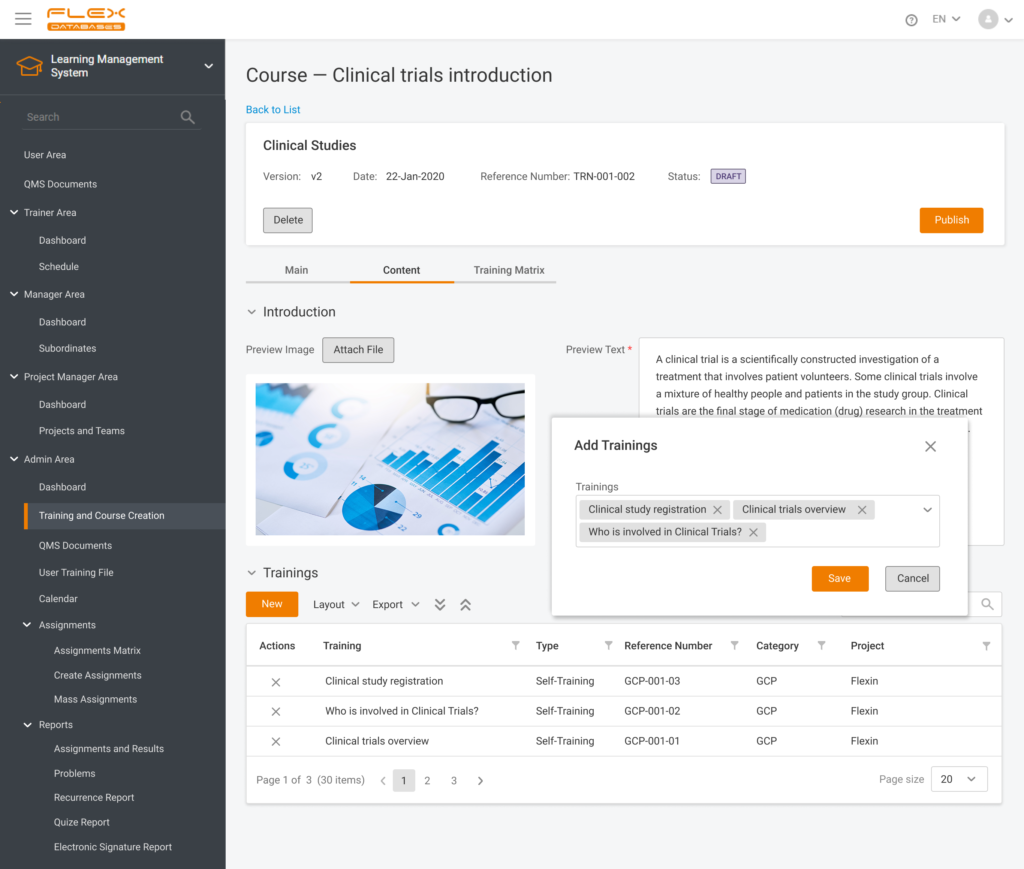
Nowadays, Learning Management System (LMS) acts as a virtual hub that helps not only with documentation, administration, reporting, tracking, delivery, and automation of training but mainly with audits and SOPs management.
LMS has some crucial business benefits:
- Cost savings: Moving training online significantly reduces company spending on employee travel, facilities, and instructors.
- Centralized training delivery: LMS acts as a single source for content, course materials, and instructions, delivering a consistent training and learning quality to all employees. Onboarding newcomers becomes very simple, as well as acquisitions of new businesses.
- Tracking of employee progress and performance: LMS allows the company to get reporting on an overall or user level basis. It is possible to track easily goal progress, knowledge gains, and more.
- Regulatory compliance: Thanks to LMS, the update of QA documents becomes very simple. Audit or inspection is never a problem and does not require substantial preparation.
There are tons of things to consider when it comes to choosing the right Learning Management System.
Let’s take a quick look at some of them:
- LMS allows automatic training assignments, which helps efficiently scale and integrate new companies or teams into corporate culture.
- LMS acts as an online library: SOPs, company templates, CVs, job descriptions, training files, and certificates are stored in one place.
- LMS has different training options: trainer-led, self-training, and a course.
- LMS supports various content formats, including videos, presentations, links, documents attachments, SCORM, and interactive quizzes, making training easy to go through.
- LMS provides deep business intelligence reporting for all training matters – besides all basic reports you can even see how long it took to answer a specific question in a training quiz.
- Access for different levels of employees and external users – QA view, training manager view, line manager view, project manager view, and employees view.
- A compliant e-signature is available to sign the documents and confirm that training has been passed.
- Flexible notifications allow not to miss training, review, and update an SOP.
Flex Databases Learning Management System covers all these features and many more. Any audit is never a challenge. Training and SOP matrixes are maintained automatically. Training files are audit-ready and exportable in one click.
See for yourself – check out our product page or request a free demo, where we will demonstrate all the capabilities of our LMS.
You can contact us through a button on top of the page, via the form below, or simply send a request to contact@flexdatabases.com.



