




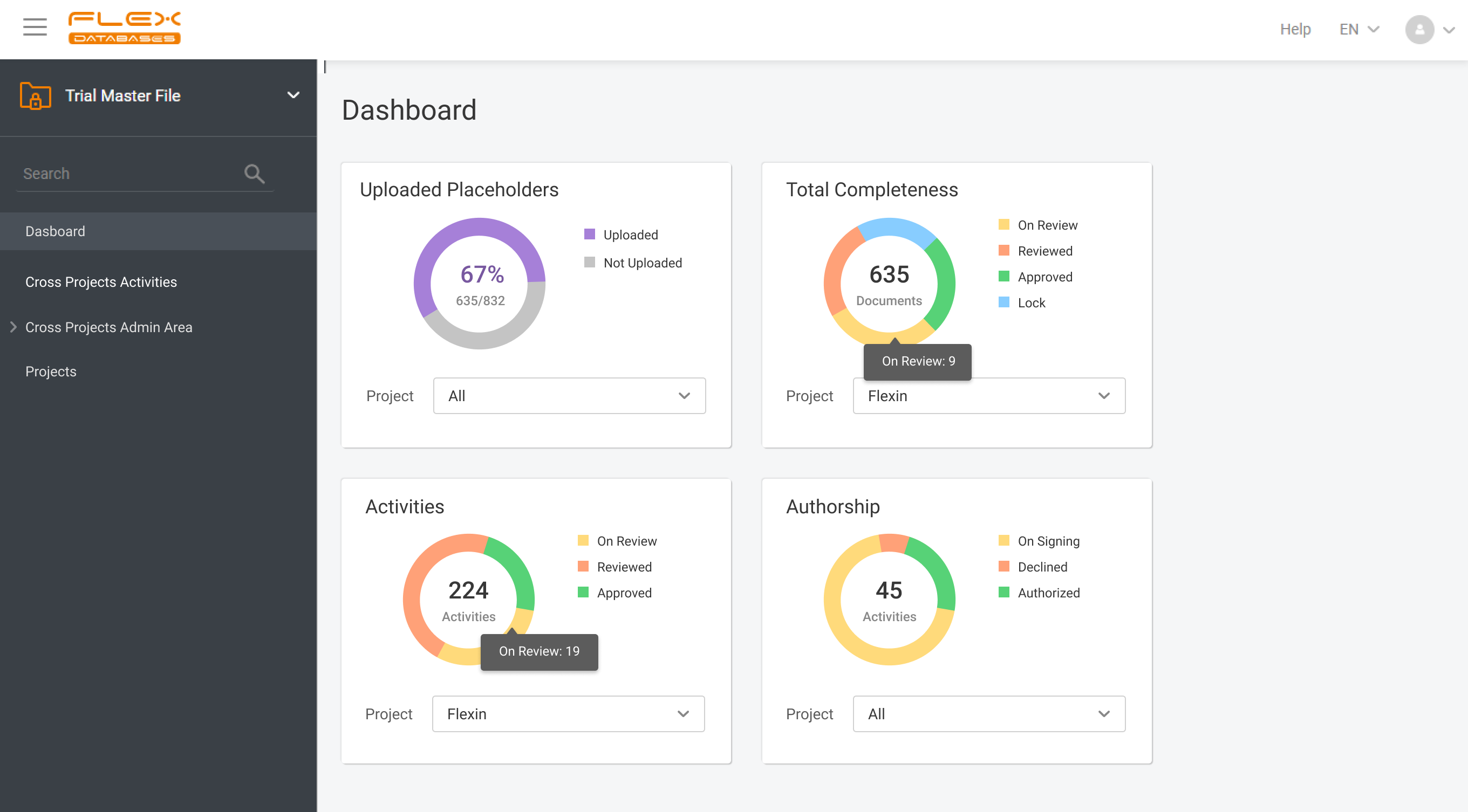
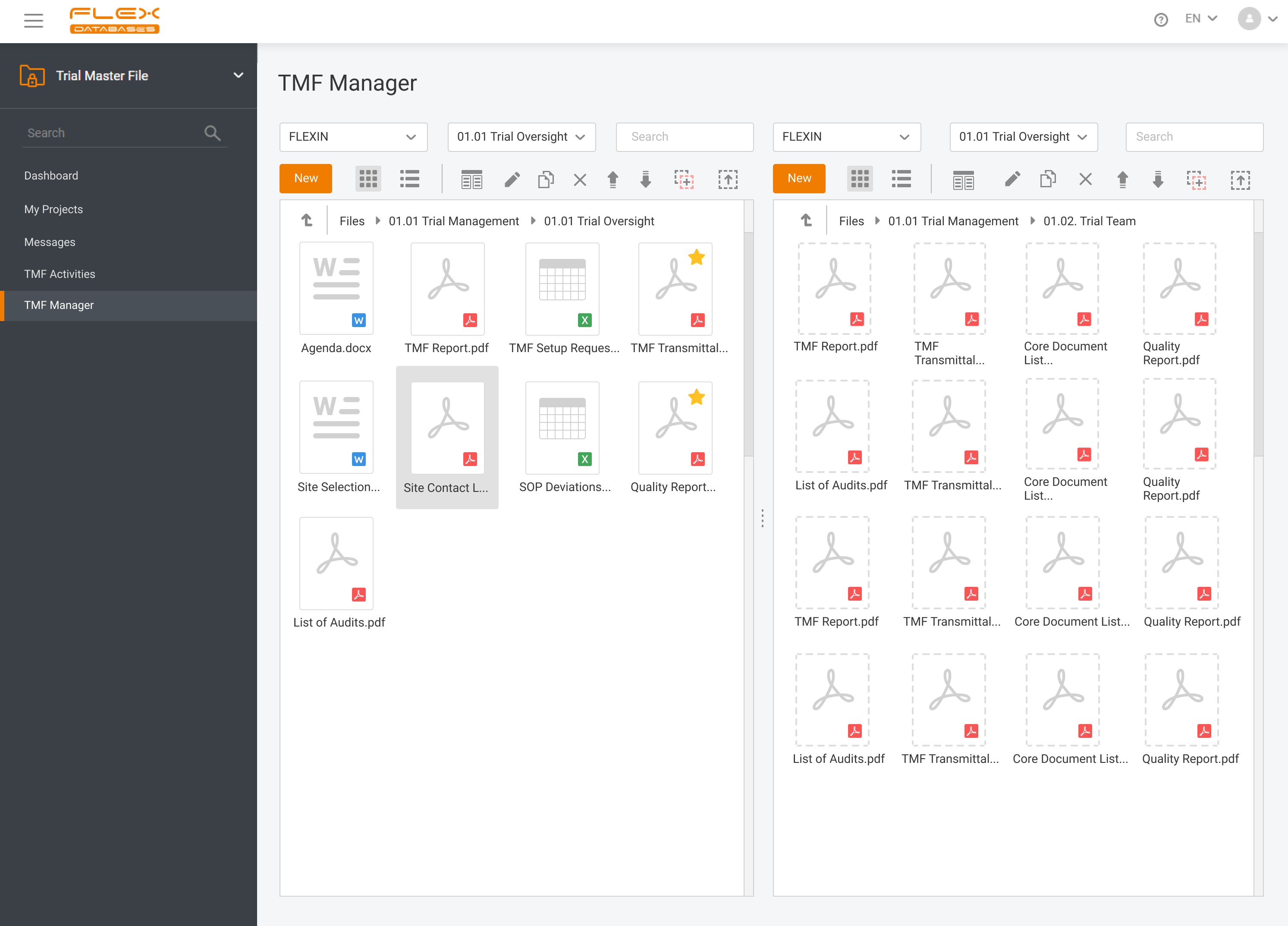
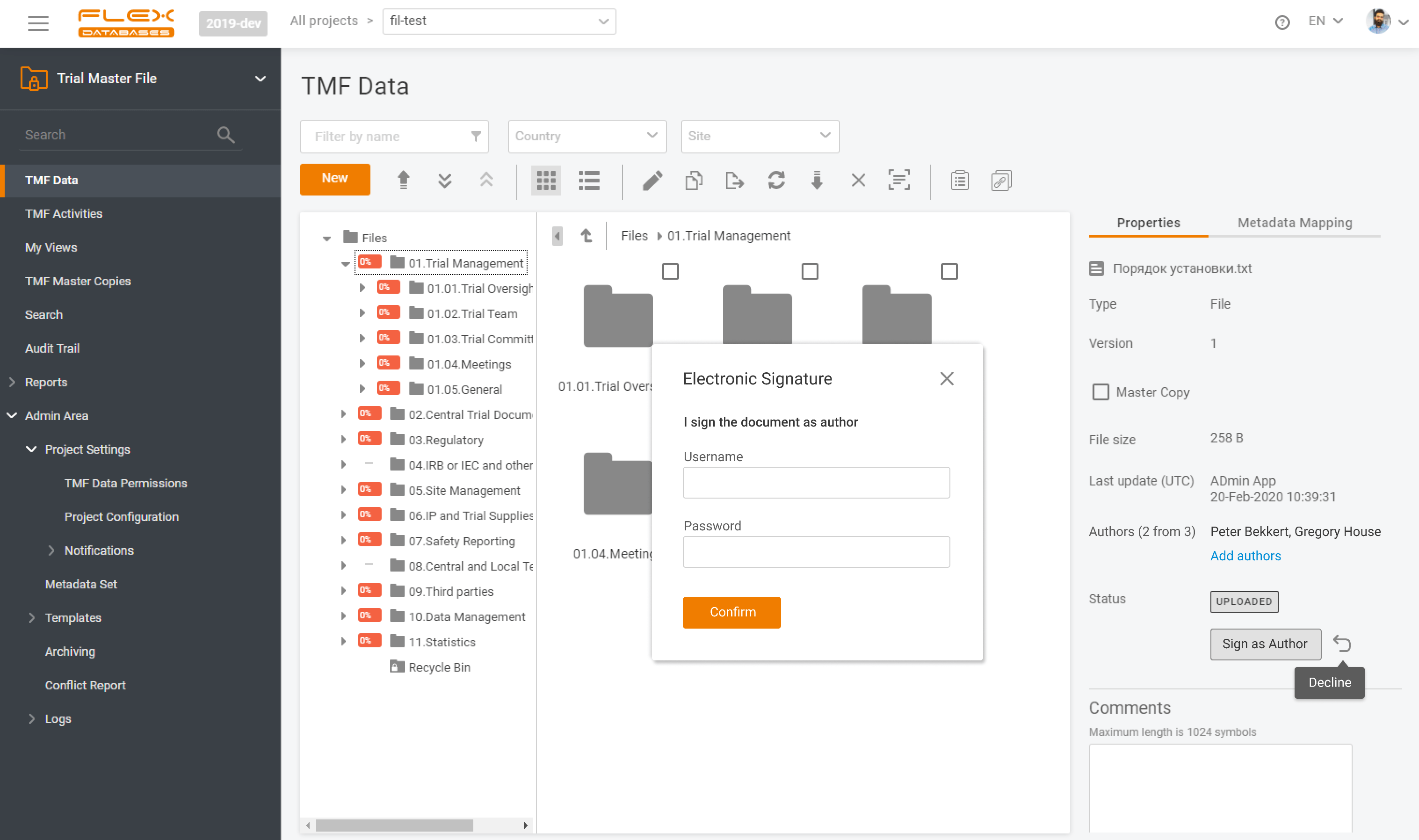
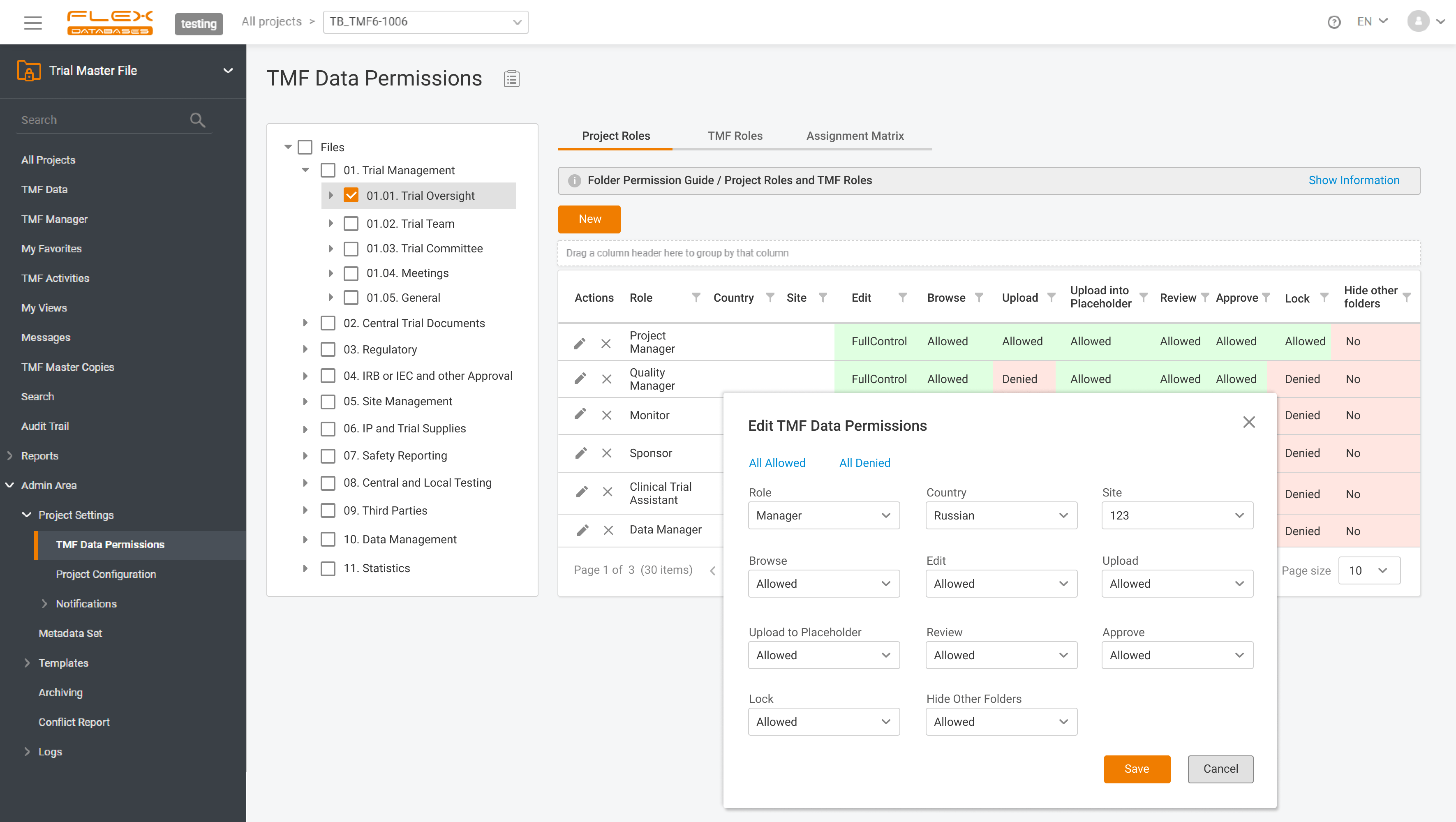
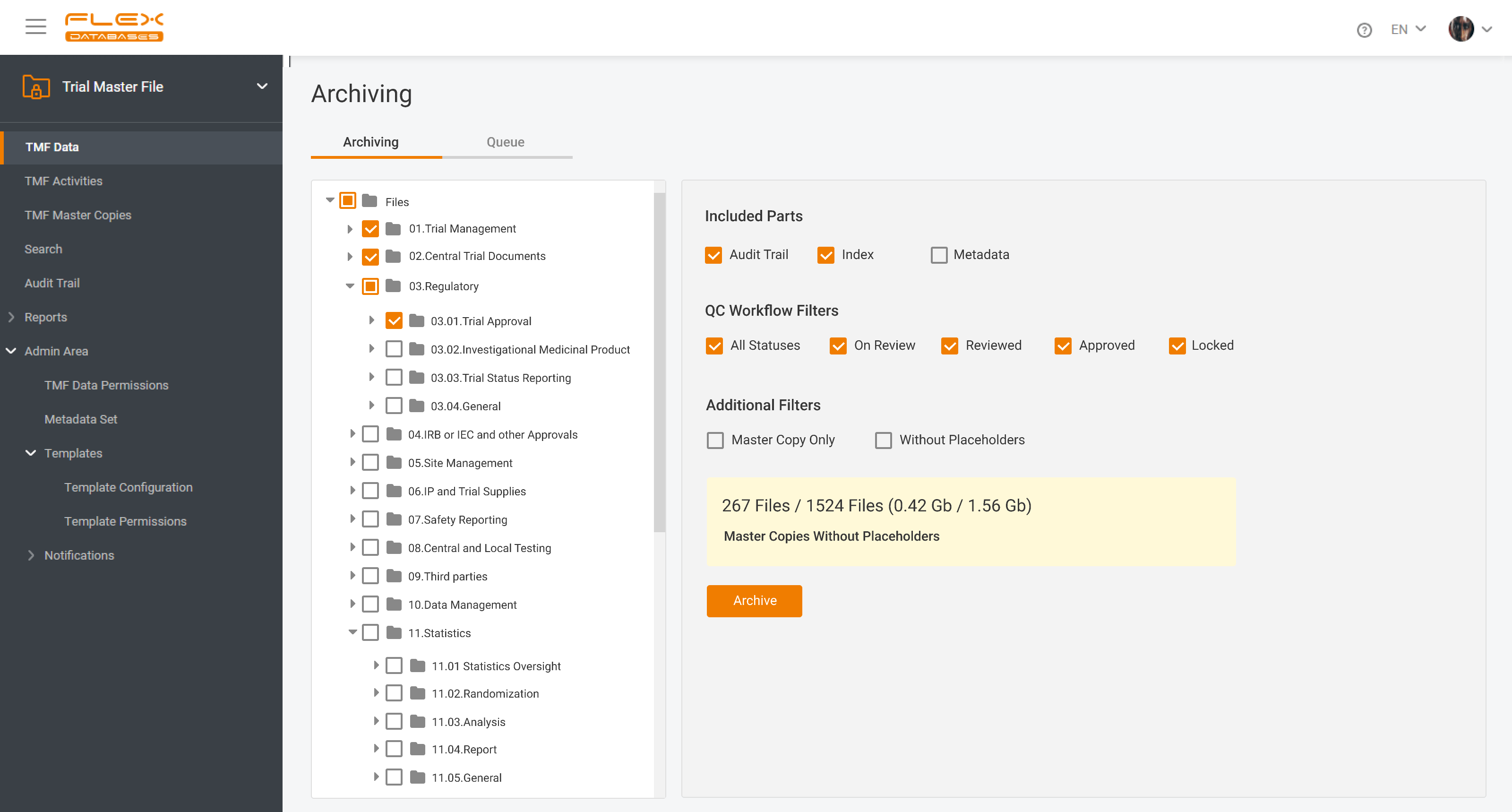
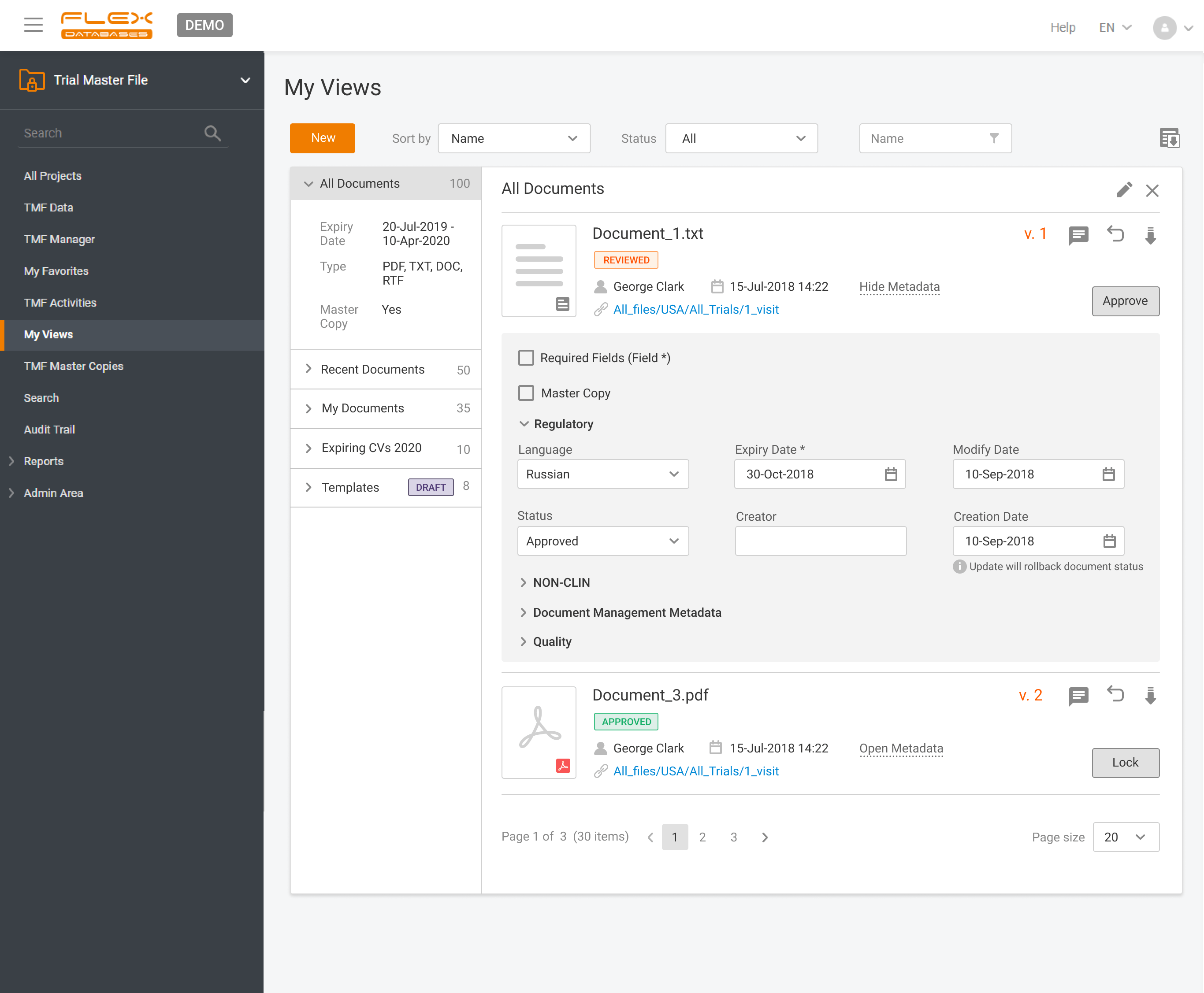
eTMF – complete 24/7 visibility into all your trial documentation





Drop us a line and we be happy to answer all your questions
Clients
Daily users
Documents
Clinical trials
Countries
Many clinical trial teams still rely on a legacy TMF – whether that means physical binders or outdated digital folders lacking structure and traceability. These systems often fall short in today’s increasingly regulated, fast-paced environment, making oversight and compliance more difficult. That’s why more sponsors and CROs are transitioning to a modern eTMF. A modern […]
An electronic Trial Master File plays a central role in maintaining oversight, ensuring regulatory compliance, and supporting successful inspections by health authorities. An efficient eTMF is no longer optional – it’s essential. Choosing the right eTMF model for your clinical studies directly impacts how effectively your teams can manage documentation, maintain version control, and collaborate […]
A mid-sized CRO managing 9,500 TMF documents annually faced increasing challenges with filing and metadata assignment. Each document required an average of 5–6 minutes to process manually. The Problem Manual document upload and metadata entry amounted to: This process also posed risks of human error and inconsistency. The Solution The CRO implemented an AI-powered eTMF […]
Alithia Life Sciences is a full-service Contract Research Organization dedicated to deliver high-quality clinical trial management solutions. With a strong focus on efficiency, compliance, and operational excellence, Alithia partners with pharmaceutical and biotech companies to ensure the seamless execution of clinical trials. To further enhance its document management and trial oversight, the company selected Flex […]
Get in touch to discuss compliance, implementation, demos, pricing
We are here for all of your questions! Tell us more about yourself and we will organize a tailored live demo to show how you can power up your clinical trials processes with Flex Databases.