




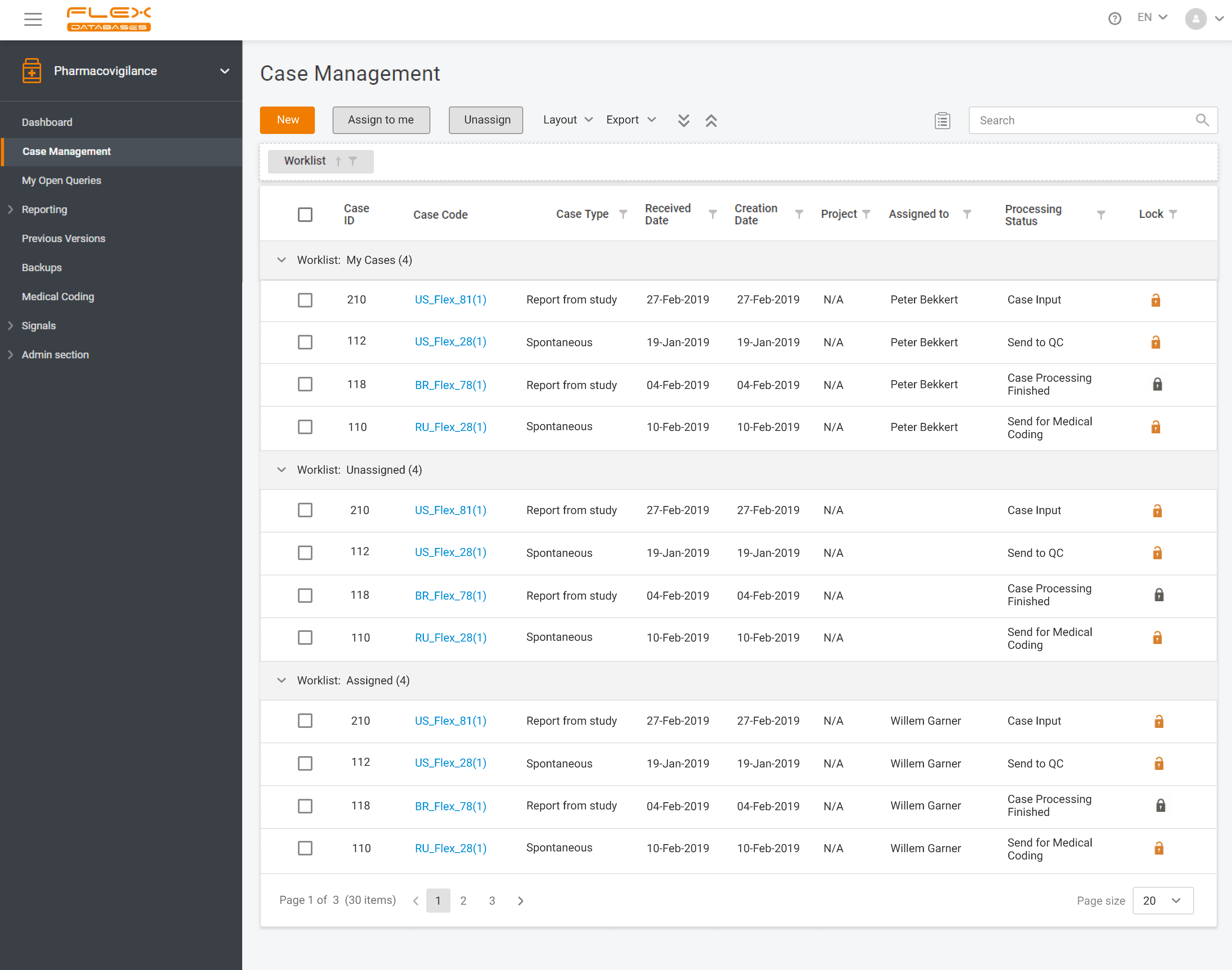
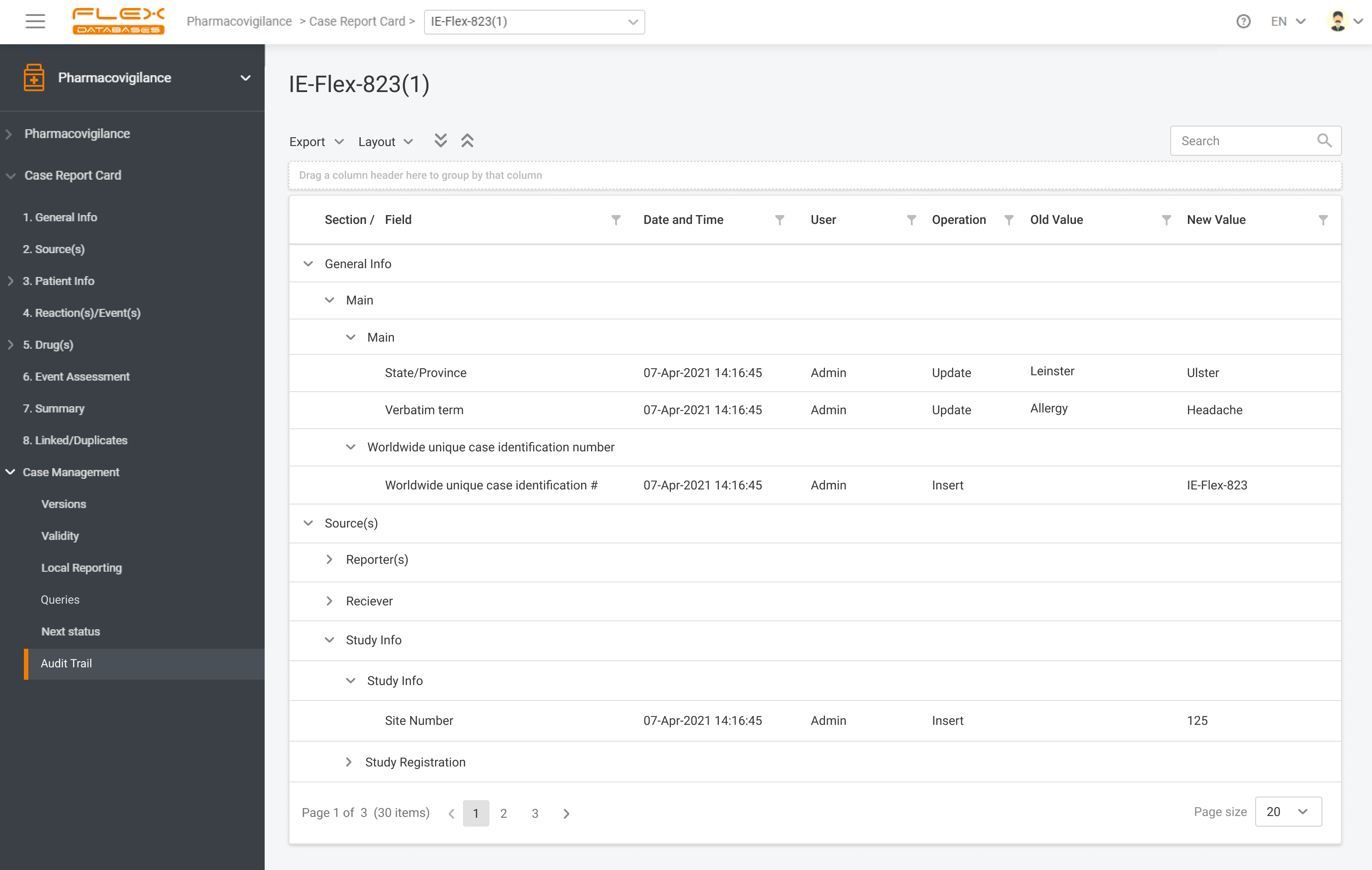
Flex Databases Pharmacovigilance is a comprehensive drug safety database that ensures robust and compliant pharmacovigilance activities both within clinical trials and post-approval stage. End-to-end process of collecting, triage, evaluation and submission of safety data in a single point.





Drop us a line and we be happy to answer all your questions
Clients
Daily users
Documents
Clinical trials
Countries
Palobiofarma is a leading clinical-stage biotech company dedicated to developing innovative medicines. Our focus on adenosine signaling drives our commitment to scientific excellence, with six drug candidates currently in different phases of development. Among them, PBF-999 for Prader-Willi Syndrome stands out as a key project. Our mission is to advance patient care by leveraging cutting-edge […]
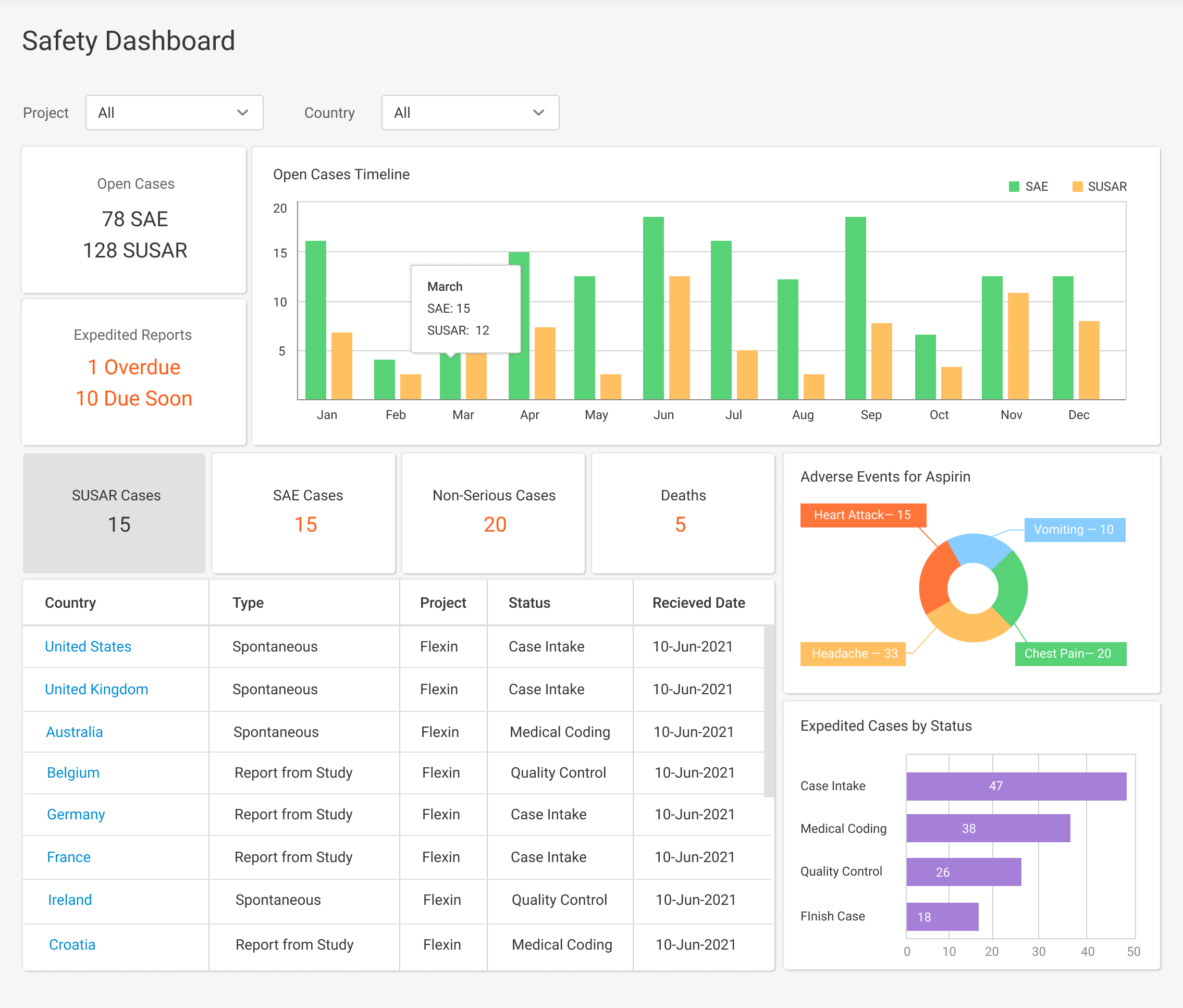
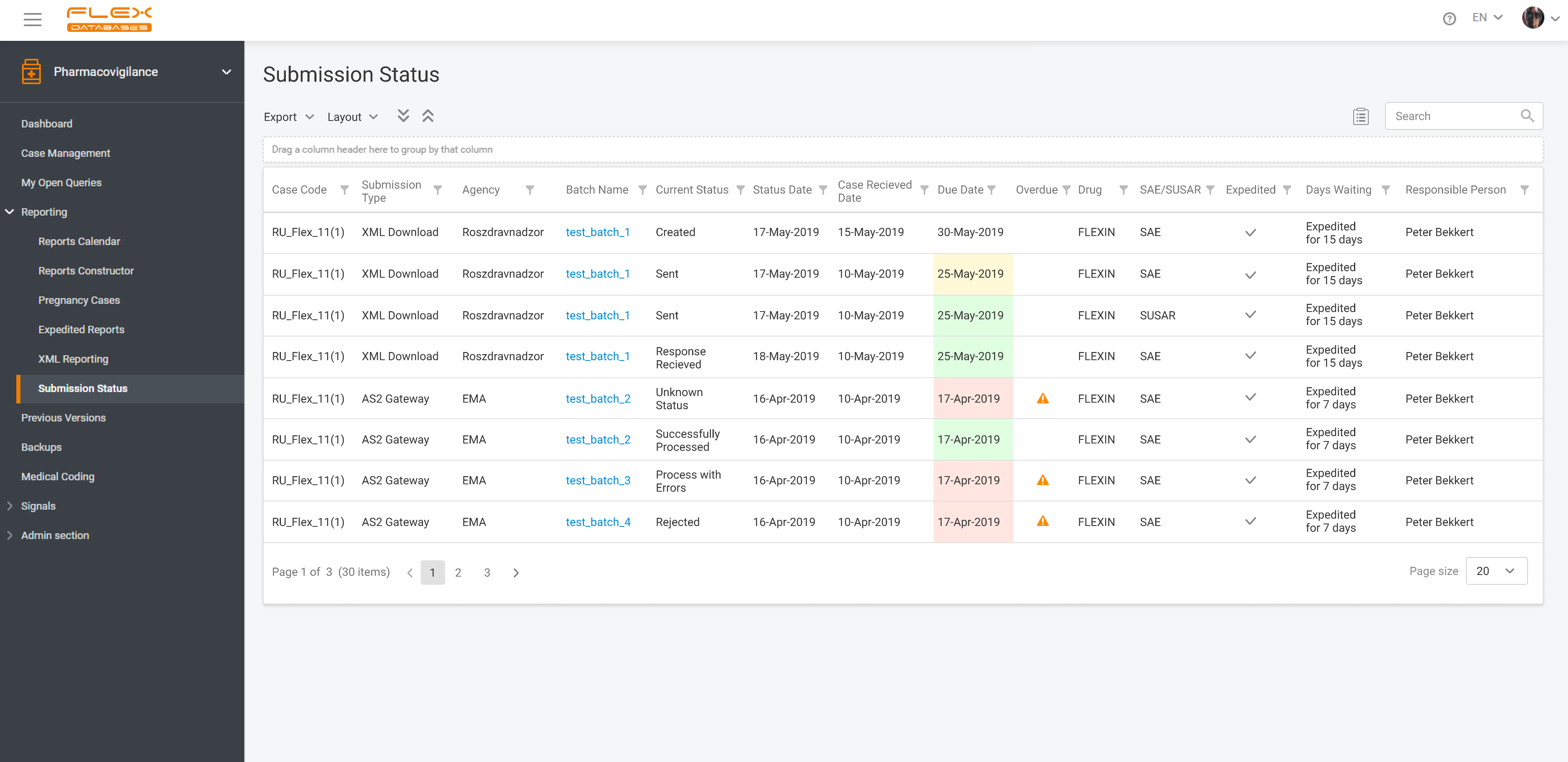
Pharmacovigilance (PV) is a critical aspect of drug safety, focusing on detecting, assessing, understanding, and preventing adverse drug reactions or other drug-related problems. As global regulatory requirements for drug safety evolve, adopting reliable pharmacovigilance software has become essential for ensuring compliance and improving operational efficiency. Selecting the right PV software can significantly impact your organization’s […]
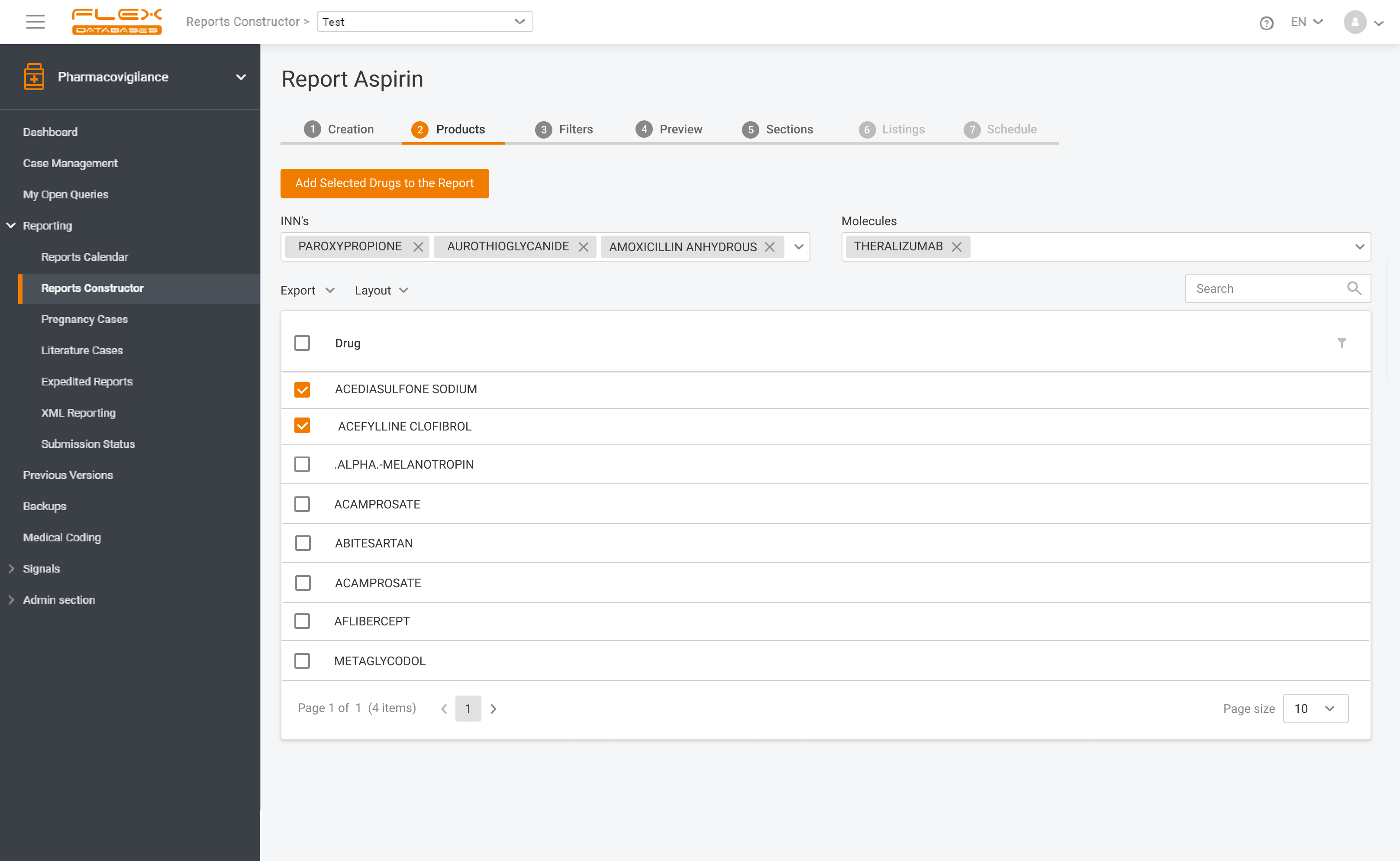
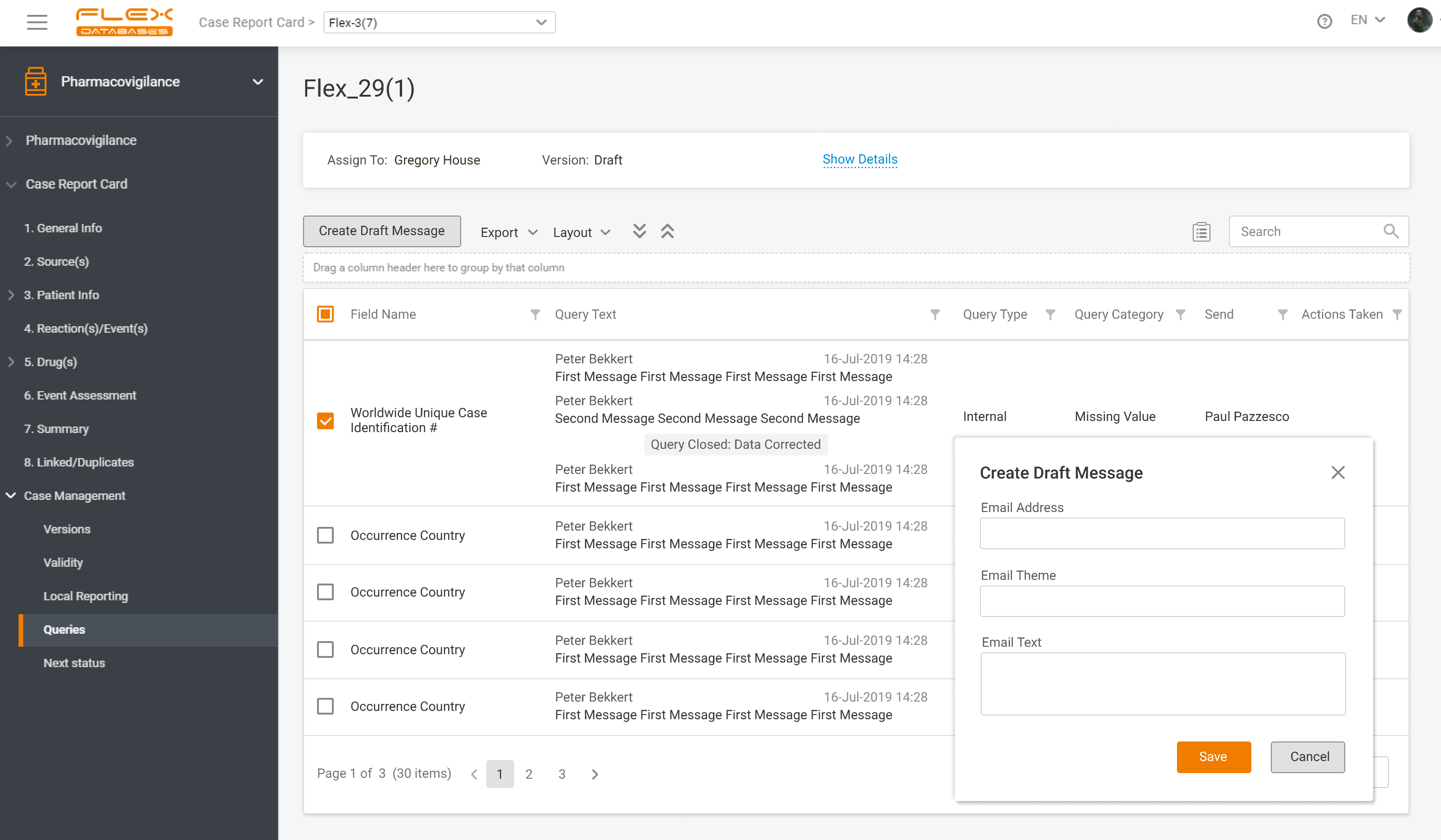
If you operate globally, you must comply with local language requirements, which also apply to pharmacovigilance reporting. Imagine that you must manage cases & report in English, Spanish, and French. How would you do that? The apparent answer many providers usually offer is multiple inputs of the same cases. Drop that! With Flex Databases Pharmacovigilance, […]
There are three big questions in any industry, including pharmaceuticals & clinical trials: Artificial intelligence is one of the new answers to all of them. Here are a few examples: When it comes to saving time, AI is here to take over data processing – fishing for answers to most minor questions in huge data […]
Get in touch to discuss compliance, implementation, demos, pricing
We are here for all of your questions! Tell us more about yourself and we will organize a tailored live demo to show how you can power up your clinical trials processes with Flex Databases.