




Manage all your documents in a smart way – have them organized, easily accessible, and ready to use.





Drop us a line and we be happy to answer all your questions
Clients
Daily users
Documents
Clinical trials
Countries
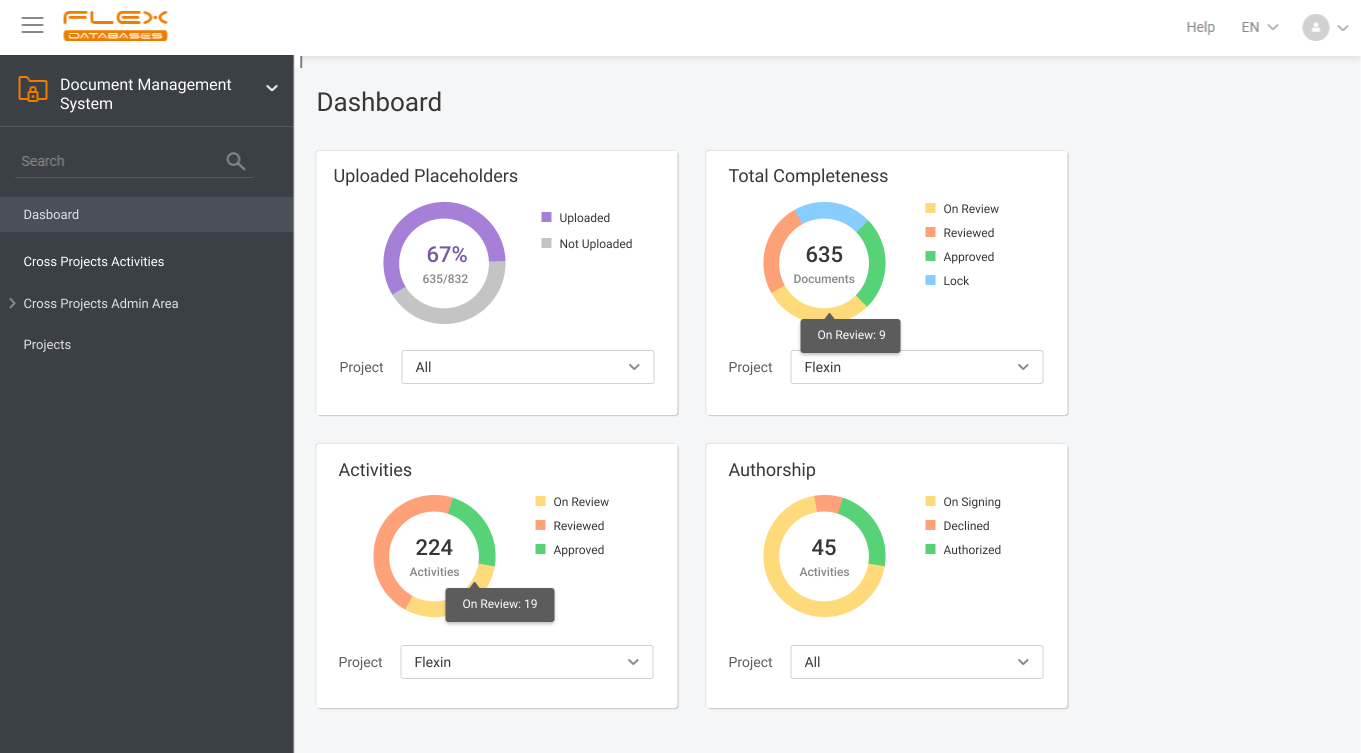
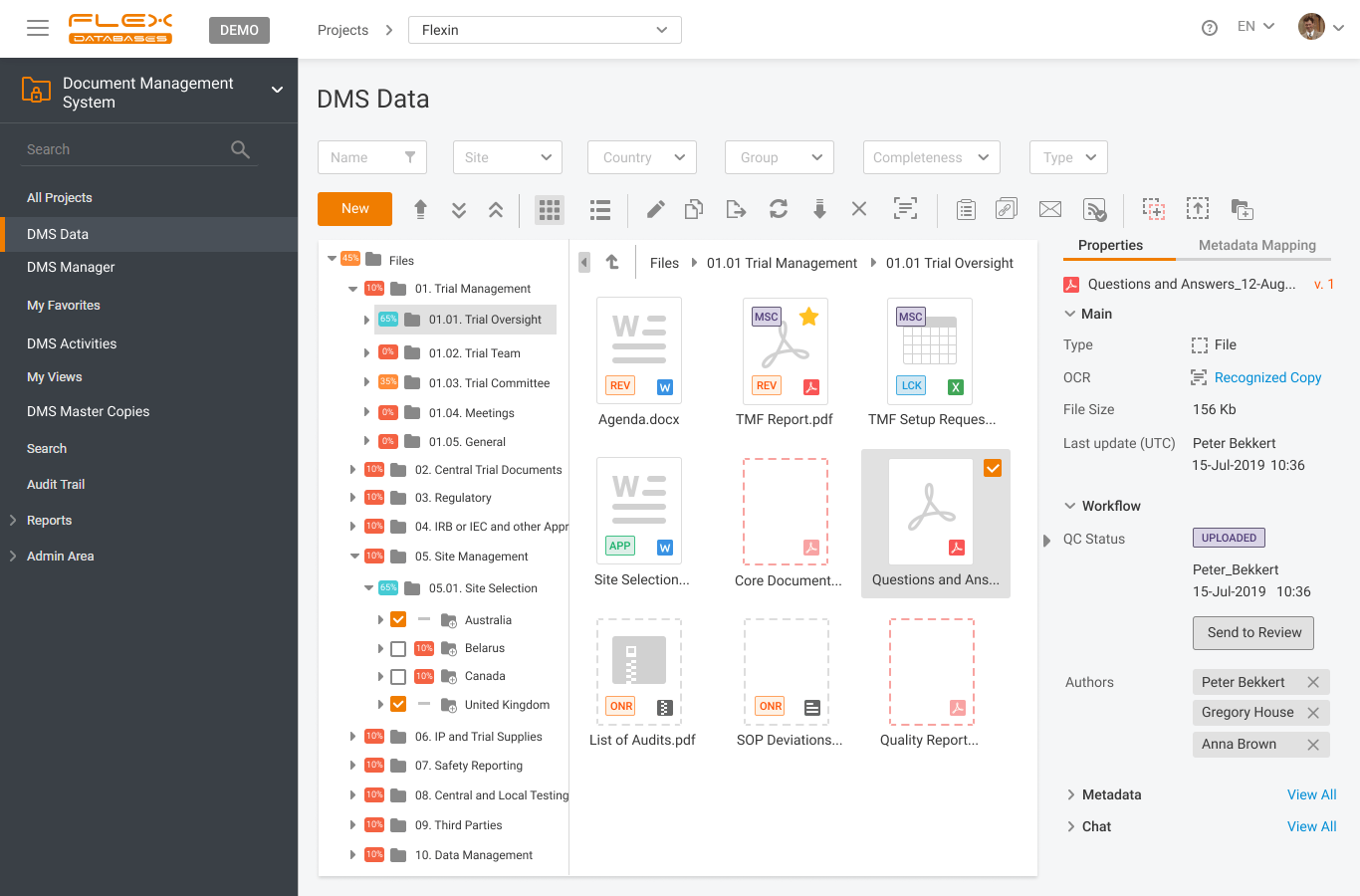
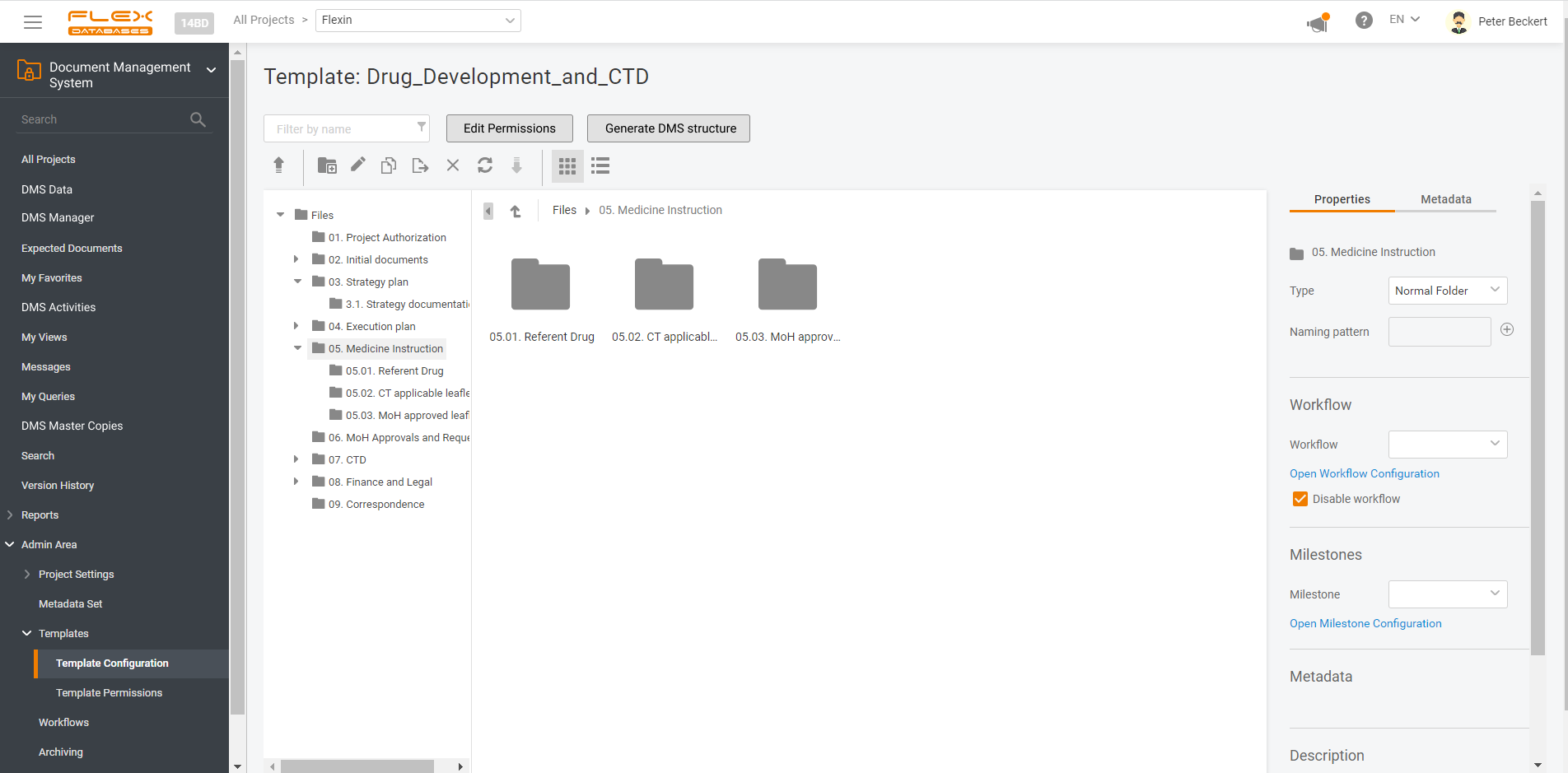
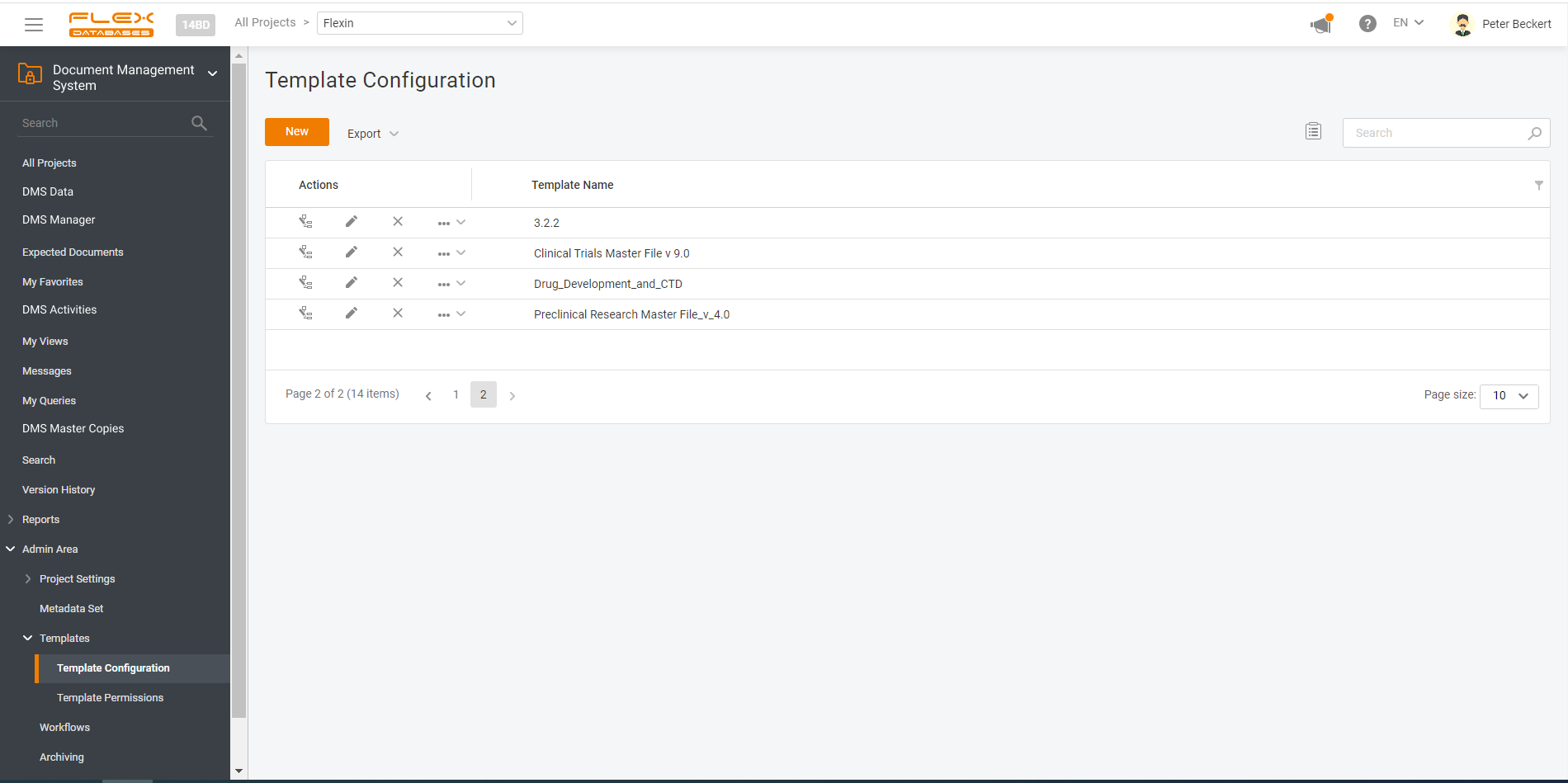
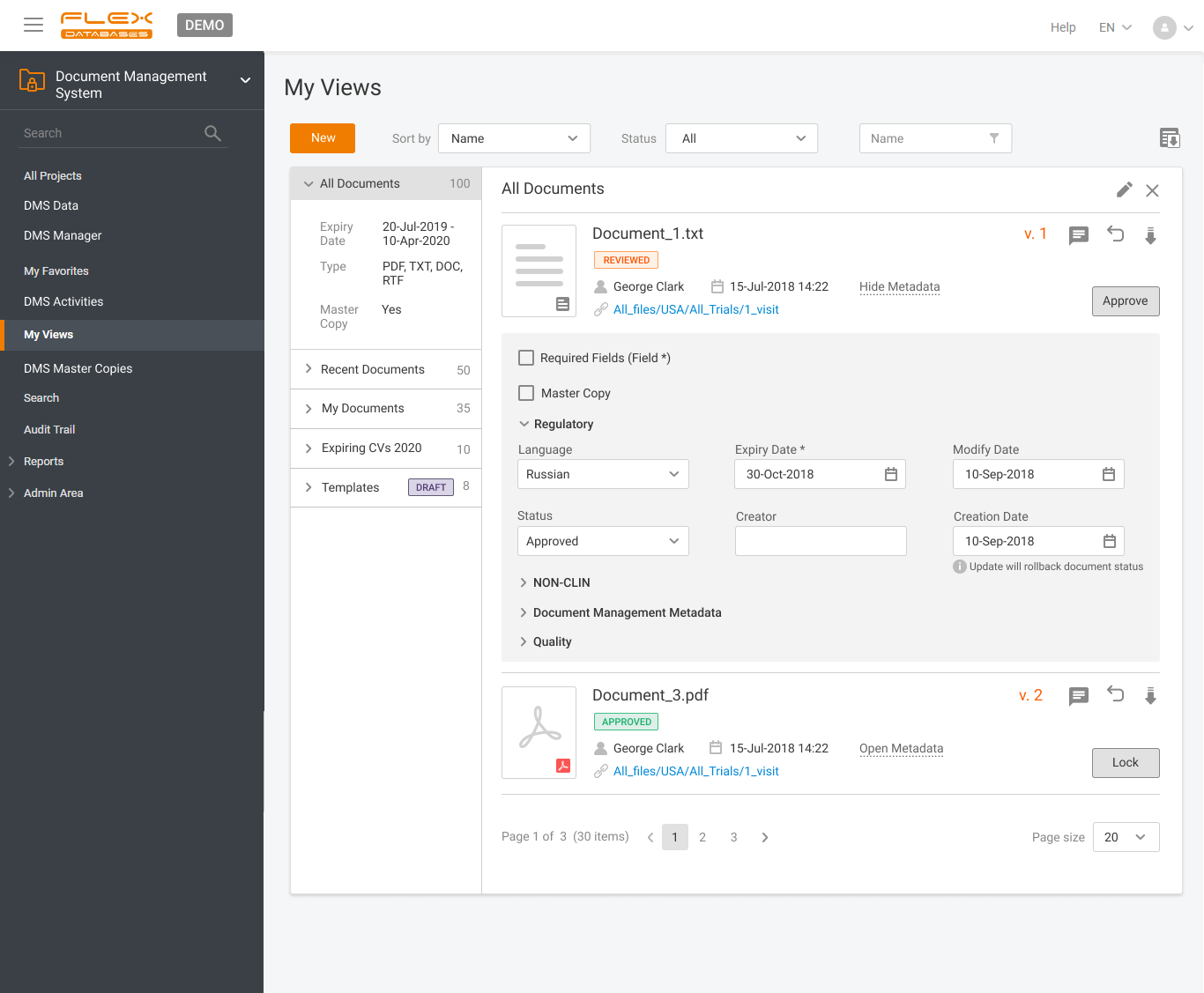
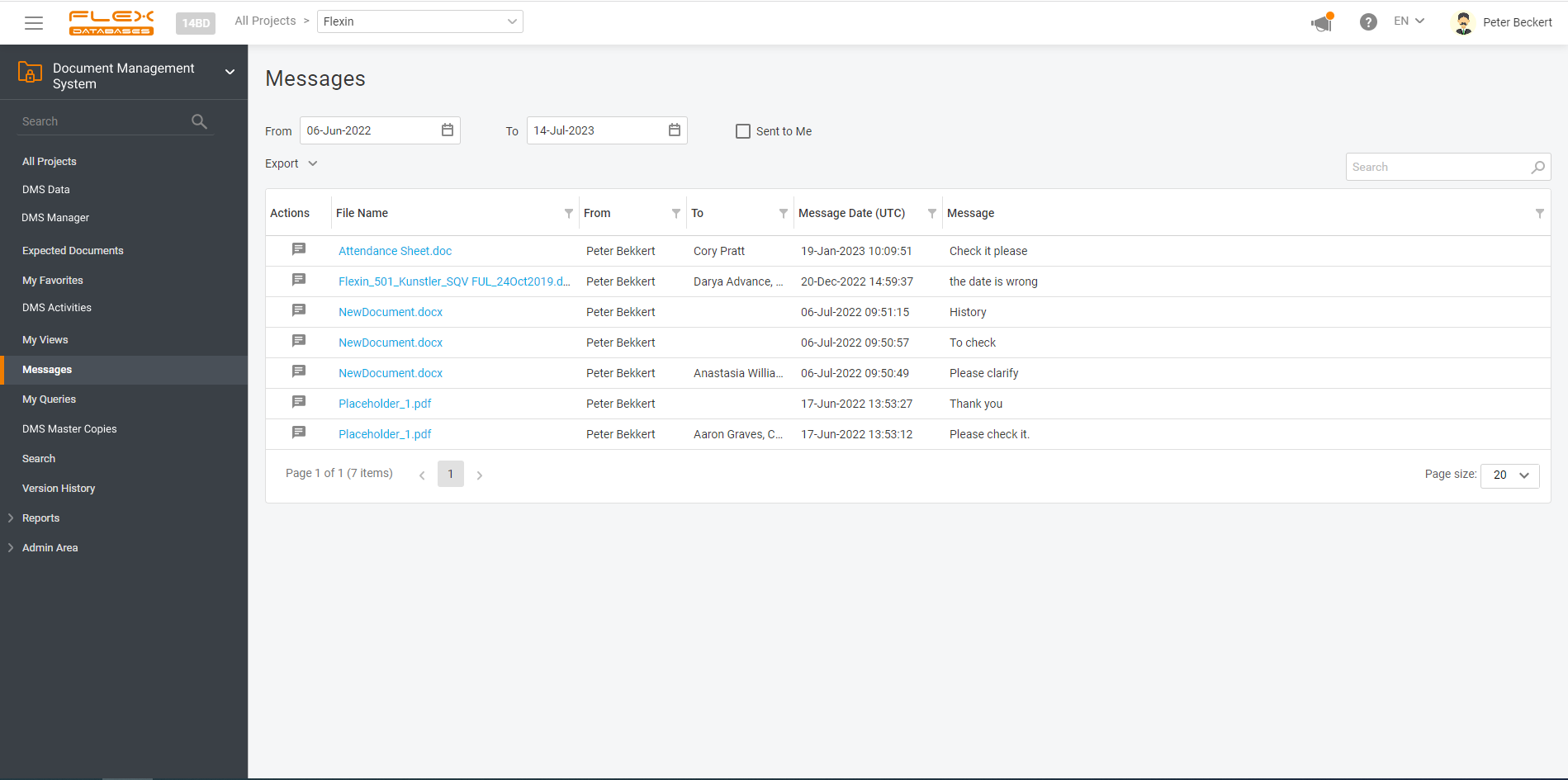
Document management & storage is rarely considered as something complicated, however, it’s a backbone for everything. When it’s done wrong, you’ll experience process delays, incomplete or missing documents, and other data integrity & completeness issues. Flex Databases DMS is done exactly right – and here’s a quick overview of manager & user processes, that get […]
Get in touch to discuss compliance, implementation, demos, pricing
We are here for all of your questions! Tell us more about yourself and we will organize a tailored live demo to show how you can power up your clinical trials processes with Flex Databases.