Anything you can do, with DMS would be better – manager & user perspective
September 6, 2023
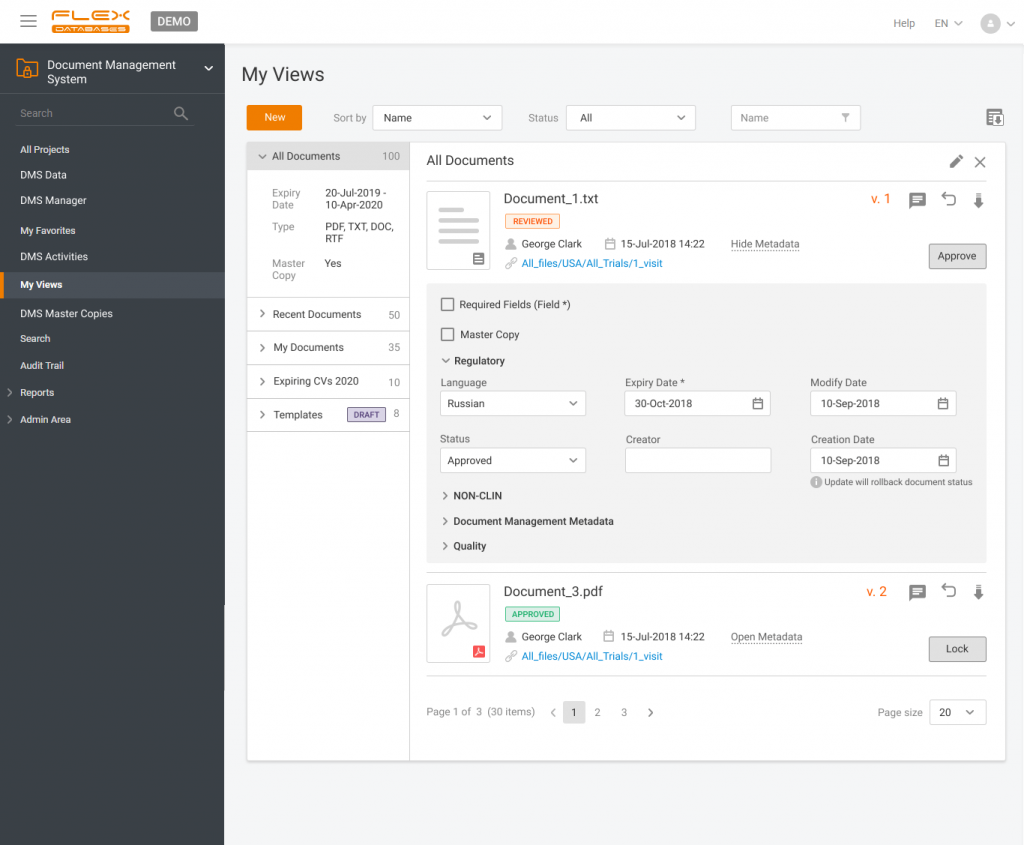
Document management & storage is rarely considered as something complicated, however, it’s a backbone for everything. When it’s done wrong, you’ll experience process delays, incomplete or missing documents, and other data integrity & completeness issues. Flex Databases DMS is done exactly right – and here’s a quick overview of manager & user processes, that get simply better with its implementation.
Management perspective:
A comprehensive system designed to enhance efficiency and compliance throughout your document lifecycle
- E-Signatures as an in-built part of the system
- Fully validated for regulatory compliance
- Seamless integration for data reusability
A user-friendly system that can be navigated intuitively without excessive training
- Effortless communication options
- Full document tracking and archiving
- Smart search and filtering
A ready-to-use system with extreme flexibility
- Tailored access control
- Choose from built-in or customize your structure templates
- Create your own workflow
User perspective:
Simplify your day-to-day work
- Automated to-do lists, flexible widgets, and exportable reports
- Review, approve, lock, sign, and author documents with the eSignature function
- Send documents in bulk
Have your documents in a structured manner
- Apply any in-built template or create your own
- Work with the documents straight in the system
- Search for a specific document or filter documents by status, milestone, completeness, etc.
Keep the whole team on the same page
- Differentiate the level of access rights
- Use any communication tools: comments, chats, queries
- Get any previous version of a document in seconds
As you can see, it’s rocket science – only your daily processes, but better. Want to learn more about Flex Databases DMS? Schedule a demo through the form or get in touch at bd@flexdatabases.com.



