




One system for clinical development process and beyond. Evidence-based decisions. More information – less efforts.





Drop us a line and we be happy to answer all your questions
Clients
Daily users
Documents
Clinical trials
Countries
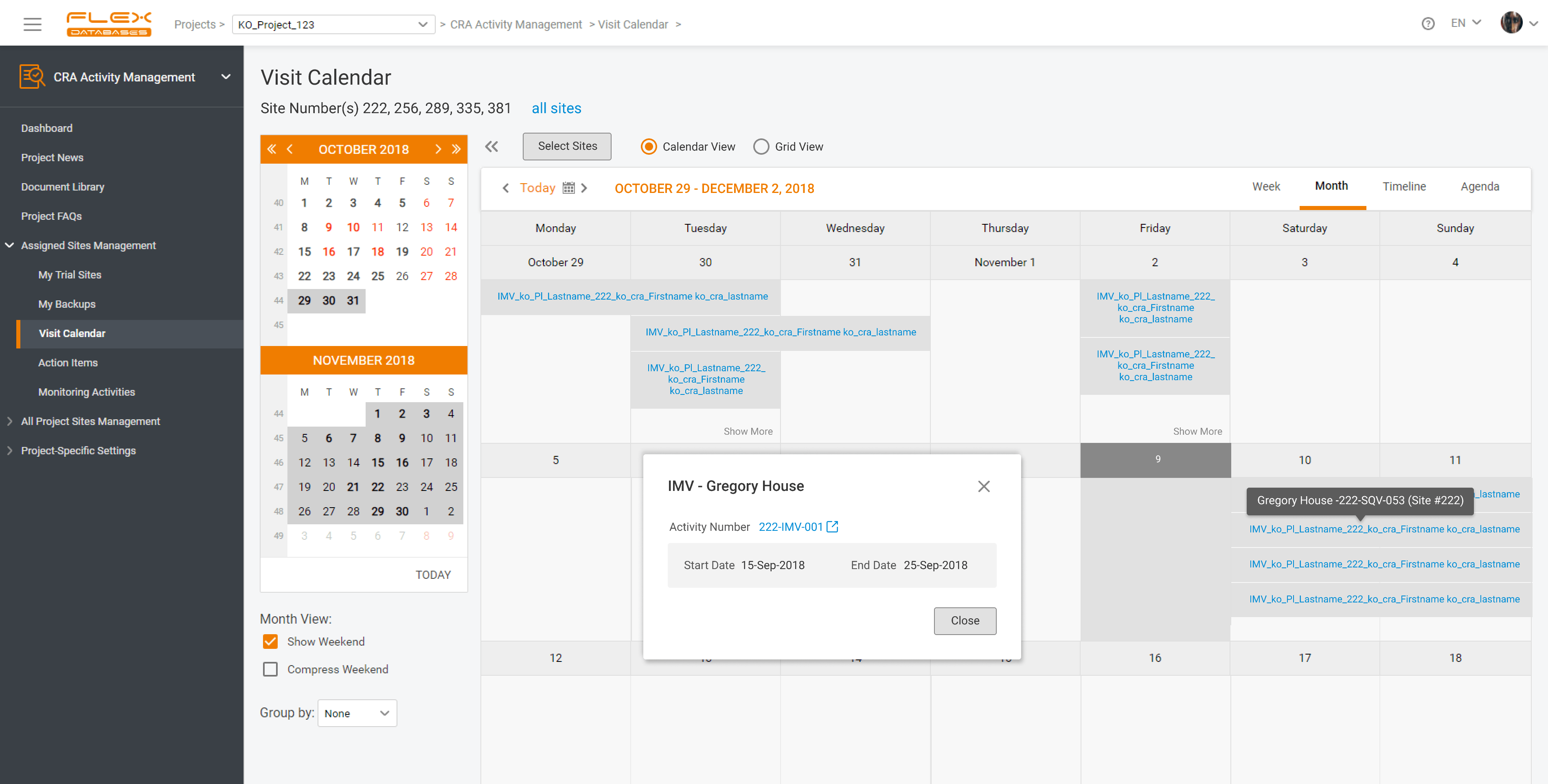
When preparing a tender for a Clinical Trial Management System (CTMS) and an electronic Trial Master File (eTMF), CROs must choose solutions that meet sponsor expectations and improve operational efficiency while keeping studies inspection-ready. The right choice affects study delivery, compliance, and client satisfaction. Below are the key factors CROs should consider. Regulatory Compliance and […]
A Comparative Look at Europe vs. US Markets In clinical operations, time is money – but how much money can technology really save you? We analyzed the return on investment (ROI) for implementing electronic Trial Master File (eTMF) and Clinical Trial Management Systems (CTMS) based on real-world benchmarks. By modeling savings from reduced manual effort, […]
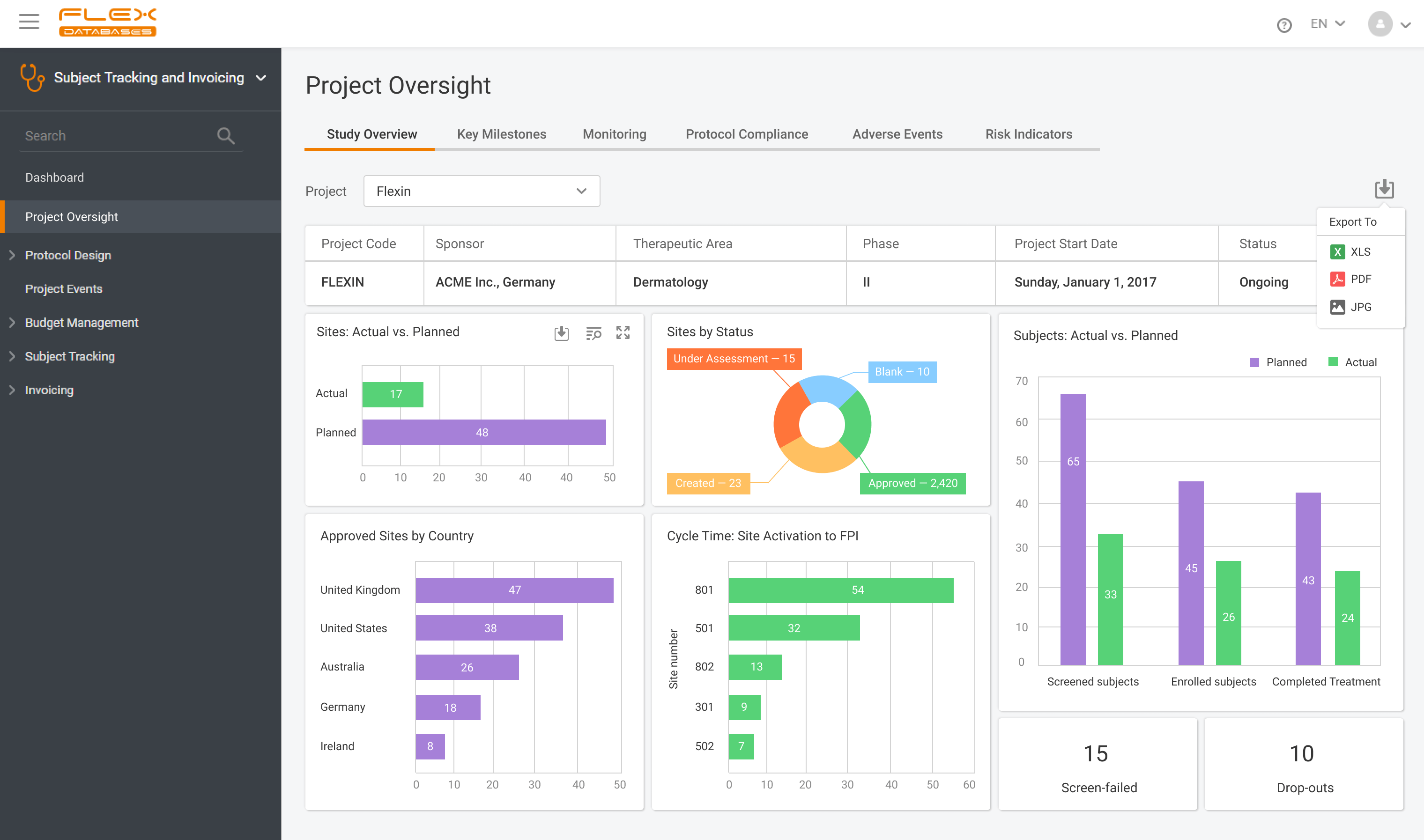
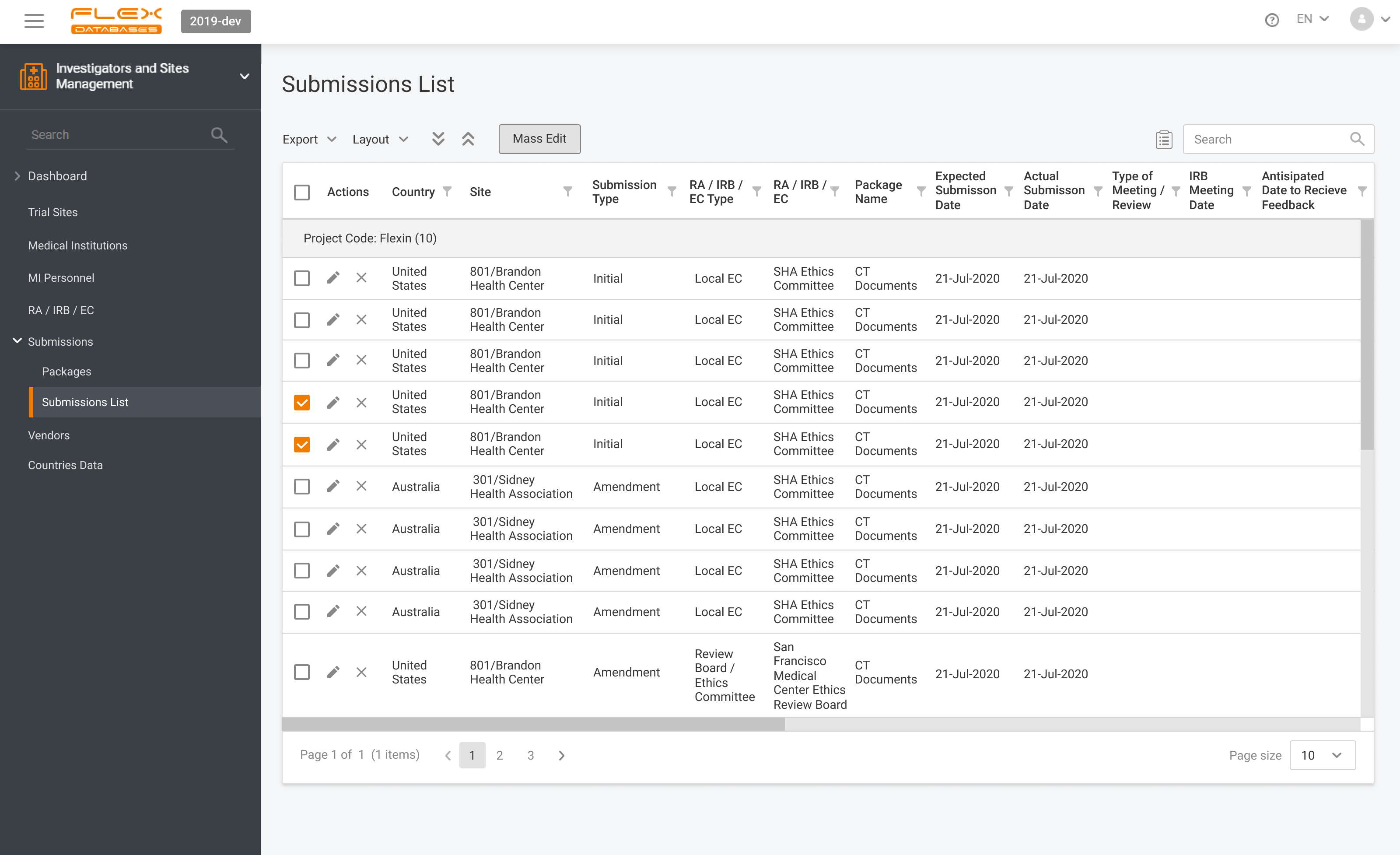
What is CTMS A CTMS – Clinical Trial Management System is a software solution that facilitates more streamlined management and monitoring of clinical trials. Clinical operations teams like project managers, CRAs, and study coordinators in pharmaceutical firms, CROs, and biotech firms use the tool to oversee everything from site selection and subject recruitment to monitoring […]
About SOLTI SOLTI is a non-profit academic research group specializing in breast cancer clinical and translational research. Based in Barcelona, the group unites over 510 professionals across 113 hospitals, including oncologists, pathologists, radiologists, and study nurses. With 42 ongoing studies and a team of 60+ staff members, SOLTI supports clinical trials throughout their entire lifecycle […]
Get in touch to discuss compliance, implementation, demos, pricing
We are here for all of your questions! Tell us more about yourself and we will organize a tailored live demo to show how you can power up your clinical trials processes with Flex Databases.