Blog Flex Databases
When preparing a tender for a Clinical Trial Management System (CTMS) and an electronic Trial Master File (eTMF), CROs must choose solutions that meet sponsor expectations and improve operational efficiency while keeping studies inspection-ready. The right choice affects study delivery, compliance, and client satisfaction. Below are the key factors CROs should consider. Regulatory Compliance and […]
A Comparative Look at Europe vs. US Markets In clinical operations, time is money – but how much money can technology really save you? We analyzed the return on investment (ROI) for implementing electronic Trial Master File (eTMF) and Clinical Trial Management Systems (CTMS) based on real-world benchmarks. By modeling savings from reduced manual effort, […]
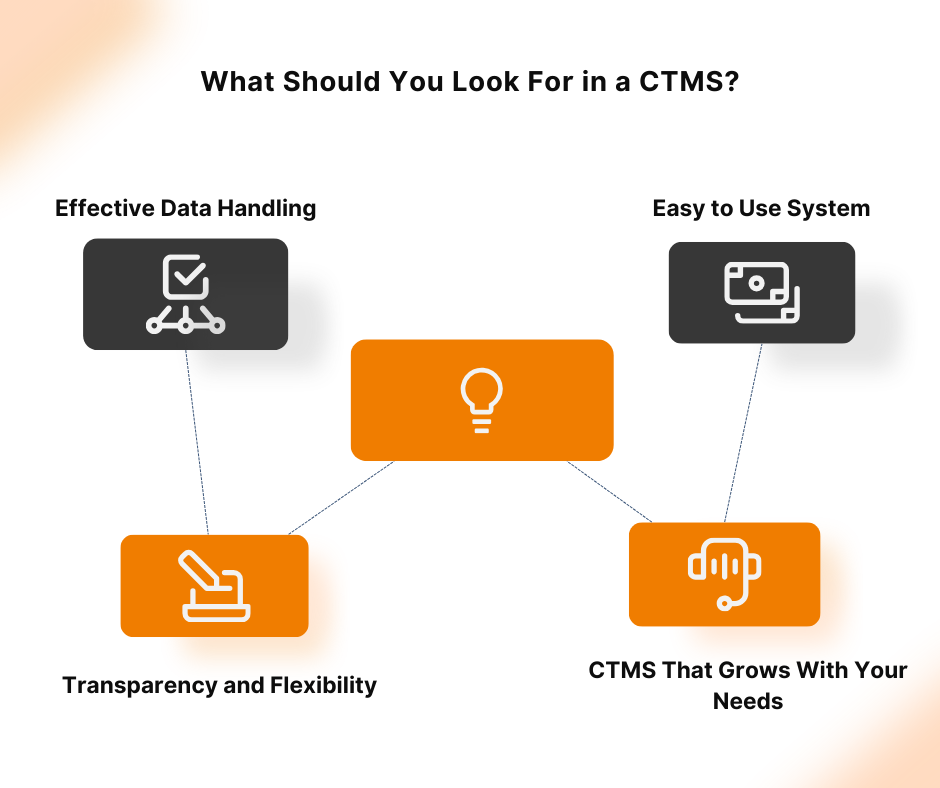
What is CTMS A CTMS – Clinical Trial Management System is a software solution that facilitates more streamlined management and monitoring of clinical trials. Clinical operations teams like project managers, CRAs, and study coordinators in pharmaceutical firms, CROs, and biotech firms use the tool to oversee everything from site selection and subject recruitment to monitoring […]
About SOLTI SOLTI is a non-profit academic research group specializing in breast cancer clinical and translational research. Based in Barcelona, the group unites over 510 professionals across 113 hospitals, including oncologists, pathologists, radiologists, and study nurses. With 42 ongoing studies and a team of 60+ staff members, SOLTI supports clinical trials throughout their entire lifecycle […]
Nowadays the relationship between sponsors and contract research organizations (CROs) has evolved. What once was a simple outsourcing arrangement has become a strategic partnership, where both parties are expected to operate with full transparency, speed, and strict adherence to regulatory standards. Sponsors are no longer satisfied with periodic updates and disconnected spreadsheets. They want real-time […]
Clinical trials are essential for developing new medical treatments, yet they often face challenges like complex protocols, regulatory compliance, and data management issues. Implementation of a Clinical Trial Management System (CTMS) can address these challenges by centralizing data, automating workflows, and ensuring compliance, thereby enhancing efficiency and reducing errors. The adoption of CTMS solutions is […]
Clinical Trial Management Systems (CTMS) and Electronic Data Capture systems are critical to the effective management of clinical trials. By integrating CTMS and EDC, organizations can streamline processes, enhance data quality, and improve overall efficiency. Here are five key tips to ensure a successful CTMS and EDC integration. Effortless Integration of Flex Databases CTMS with […]
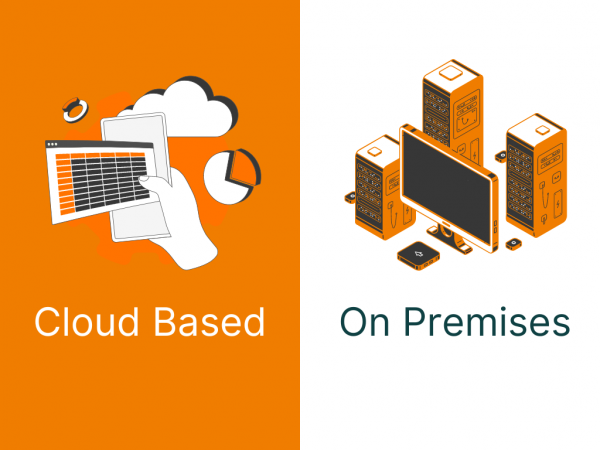
Two popular models dominate the Clinical Trial management System landscape: Cloud-based and On-premises CTMS. Both have their merits, but choosing the right option depends on several factors, including cost, scalability, security, and operational requirements. This article will explore the differences between Cloud-based Clinical Trial Management System and on-premises CTMS, helping you determine which solution best […]
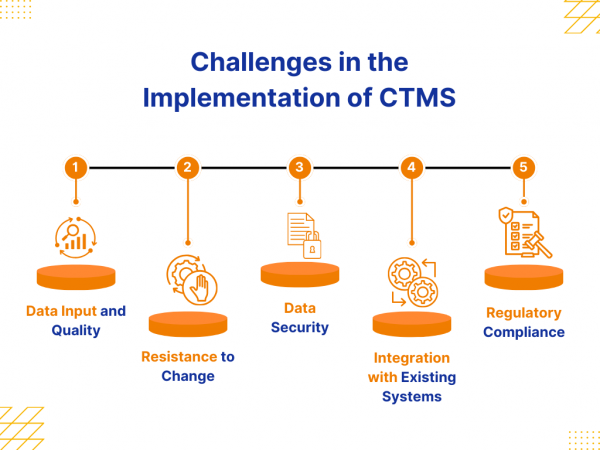
Implementation of a Clinical Trial Management System (CTMS) can streamline operations, improve data management, and enhance trial oversight. However, the process also brings a range of challenges that must be addressed to achieve a successful implementation. These challenges often stem from technical and human factors, including data quality, system integration, and regulatory demands. Identifying these […]
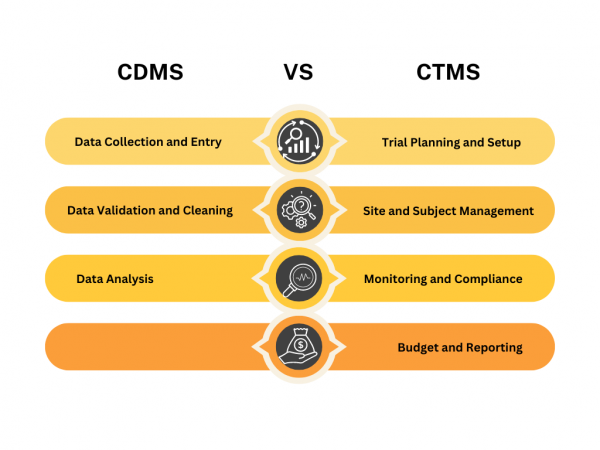
Clinical Data Management Systems (CDMS) and Clinical Trial Management Systems (CTMS) are two key tools that play crucial roles in this process. While they might sound similar, they serve distinct purposes and offer different functionalities. What is a Clinical Data Management System (CDMS)? A Clinical Data Management System (CDMS) is a specialised tool designed to […]
Clinical trials play a crucial role in medical progress, yet effective management is essential to maximize their impact. Administrators must craft a thorough strategy to enhance how trials are managed. Here are five strategies to transform clinical trial management, providing administrators with the tools and insights needed to navigate healthcare research challenges effectively. Simplify planning […]
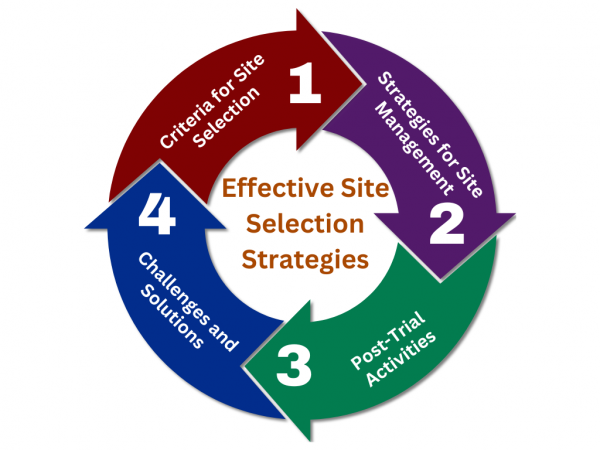
Effective site selection and management are critical components of successful clinical trials. Choosing the right locations and overseeing their operations can significantly impact the trial’s timeline, budget, and ultimately, its success in delivering reliable data. This article explores essential strategies for optimizing site selection and management, highlighting key considerations, practical tips, and the role of […]