Blog Flex Databases
When we build a product, we base its core on the challenges our customers may face and how to solve them. We step in your shoes to see things from your point of view – what you need. Here’s the list of challenges that you have, and we solve – and how exactly we solve […]
This summer ABX-CRO and Flex Databases signed a contract for a CTMS & eTMF implementation. We asked Dr. Ulrike Schorr-Neufing, Director, Qualification & Training, ABX-CRO, a couple of questions about the upcoming partnership. Why ABX-CRO decided to pick Flex Databases as a provider? After an intensive selection and evaluation process comparing different service providers ABX-CRO […]
At the very beginning of 2021 Flex Databases and German FGK CRO signed a contract for CTMS and eTMF implementation. About FGK FGK CRO is a full service contract research organization offering a complete range of clinical development and consulting services to pharmaceutical, biotechnology and medical device companies. FGK approaches each project, whether large or small, with dedicated, highly motivated, small […]
At the very end of winter, Flex Databases signed a contract for CTMS and Pharmacovigilance module implementation with Italian Human & Digital CRO Exom Group. We’ve asked Luigi Visani, MD, President & CEO at Exom Group a couple of questions about the upcoming partnership: Why did Exom Group decide to pick Flex Databases as a provider? “Since its inception, […]
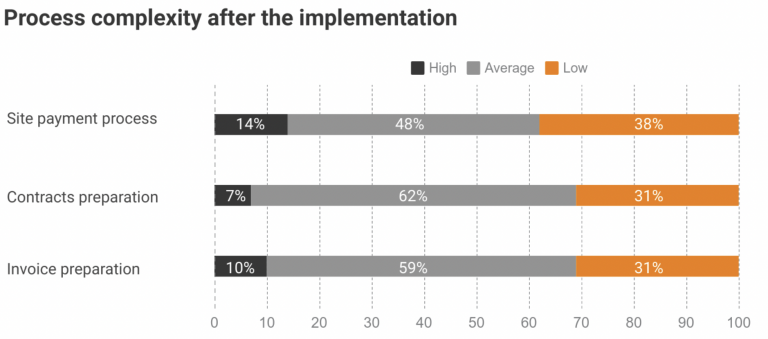
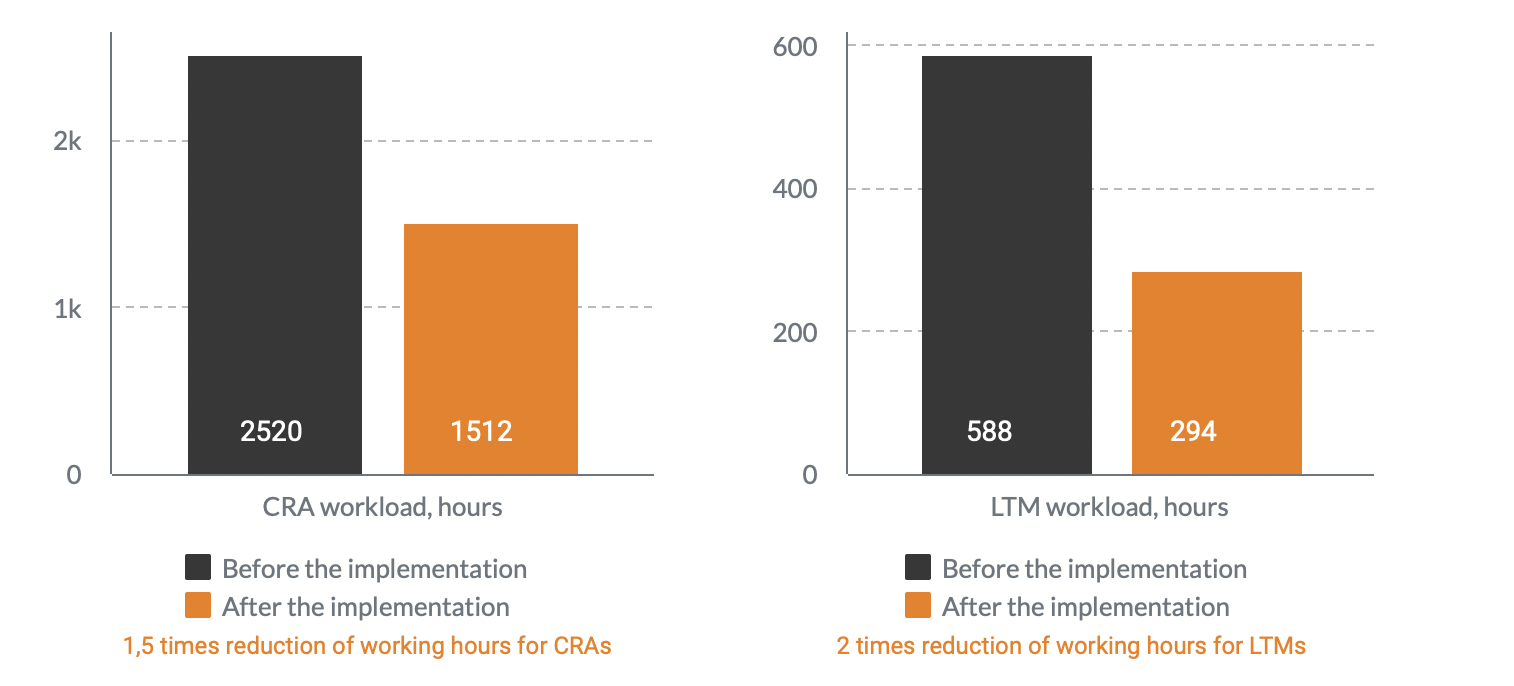
st trial sites work with no more than a 3-month operating budget. With an estimated two-thirds of trial sites falling into this category, software companies have sought to close this gap in effective financial operations with process automation and payment schedule. So CROs, pharma companies, and biotech can finally get transparency in site payments. Likewise, effective […]
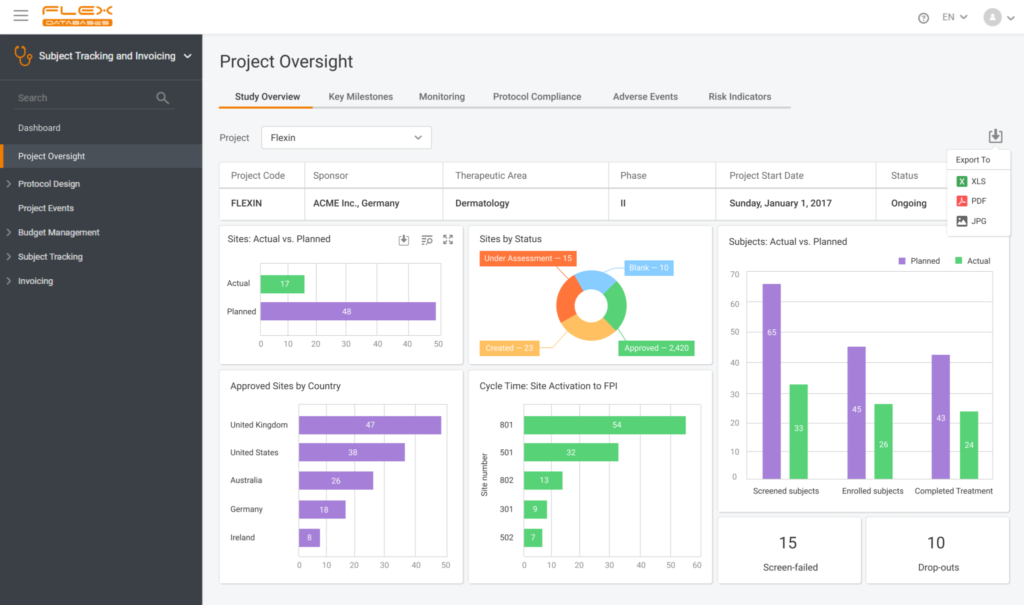
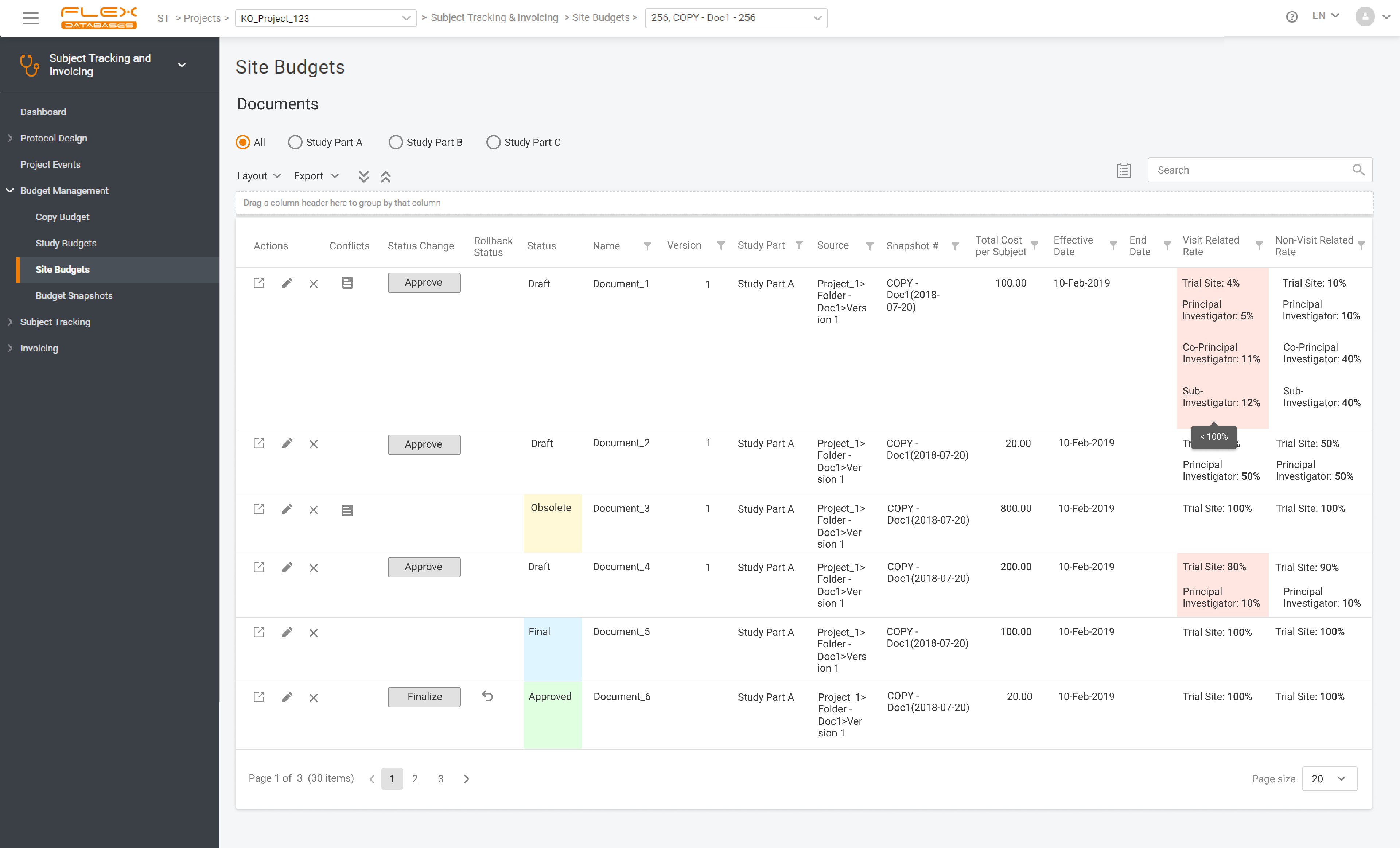
Subject Tracking & Invoicing – Flex Databases CTMS module. Use Subject Tracking & Invoicing Module to track all patients’ data and manage invoicing online. Plan and schedule patient visits efficiently. Video duration: 08:50
Bionical Emas is the only CRO to combine Clinical Development, Early Access Programs and Clinical Trial Supply, to deliver a unique, seamless approach supporting our clients to bring new medicines to patients faster. “Our partnership relationships are paramount to everything that we do, and we work closely with our clients to become an extended part of their team. We […]
United States-based Tranquil Clinical Research recently signed Flex Databases as a software provider for Clinical Trial Management System and Pharmacovigilance management solution. Tranquil’s ultimate goal is excellence in the clinical trial process and bringing trustworthy products to patients. Flex Databases CTMS and Pharmacovigilance system are well-known as unified yet flexible eClinical platform for full-cycle clinical trial management. Baseline implementation takes […]
This June Assign Data Management and Biostatistics – Assign DMB chose Flex Databases as a provider for CTMS, eTMF, and Pharmacovigilance management systems. We’ve asked Dr. Anton Klingler, CEO Assign DMB and Mag. Sabine Haid, Lead Project Manager, a few questions about the upcoming partnership: Why Assign DMB decided to pick Flex Databases as a provider for […]
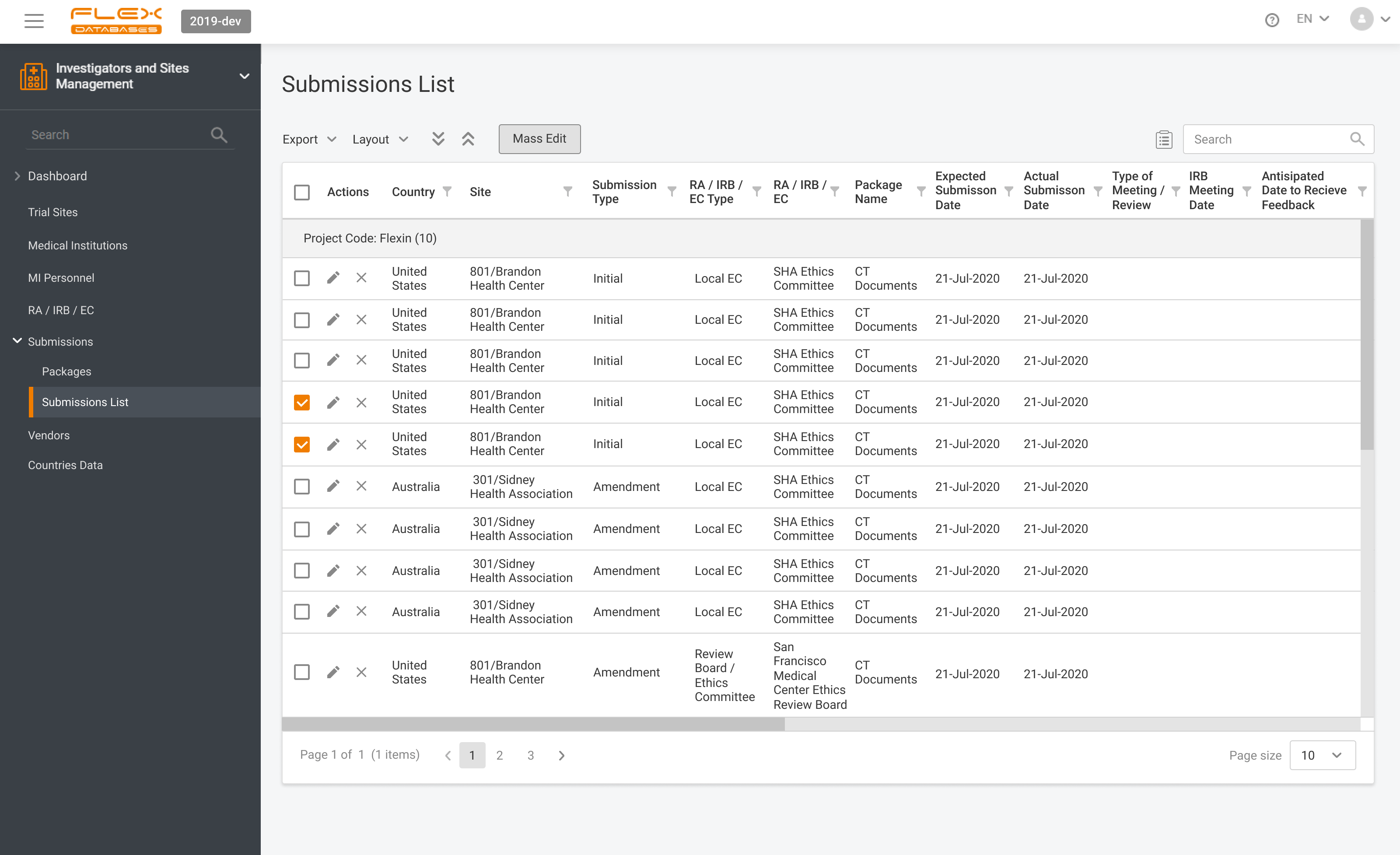
Investigators & Sites Management – Flex Databases CTMS module. Investigators & Sites Management Module helps to organize all information on investigators, sites, vendors, therapeutic areas, IRB/LECs, etc. It provides easy search capability for feasibility assessments and other needs. Video duration: 06:25
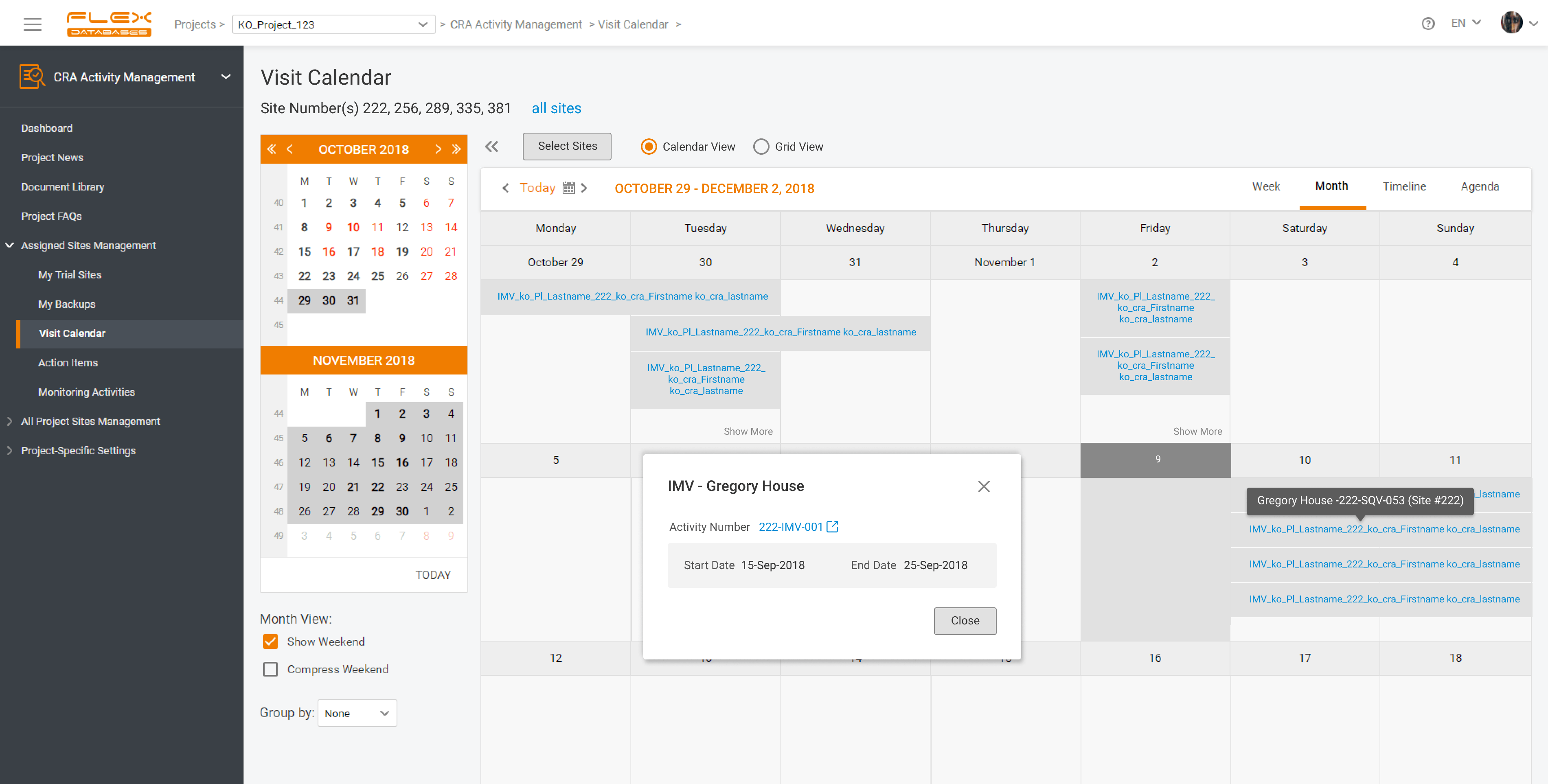
If we imagine clinical trials as a human body, Clinical Research Associates would be the blood cells. They travel a lot across the whole organism, delivering data oxygen to all organs and body parts. If something stops the proper functioning, it may result in global failure. That’s the reason why we developed CRA Activity Management module – […]
About Novo Nordisk Novo Nordisk is a global healthcare company, founded in 1923 and headquartered just outside Copenhagen, Denmark. Their purpose is to drive change to defeat diabetes and other serious chronic diseases such as obesity, and rare blood and rare endocrine diseases. Project background Most trial sites are working with no more than a […]