Blog Flex Databases
A Clinical Trial Management System (CTMS) is software designed for the Life Science industry. It helps manage processes and handle substantial amounts of data related to clinical trial management and clinical study management. CTMS typically handles the planning, preparation, execution, and reporting of clinical trials. It focuses on maintaining updated contact information for participants and […]
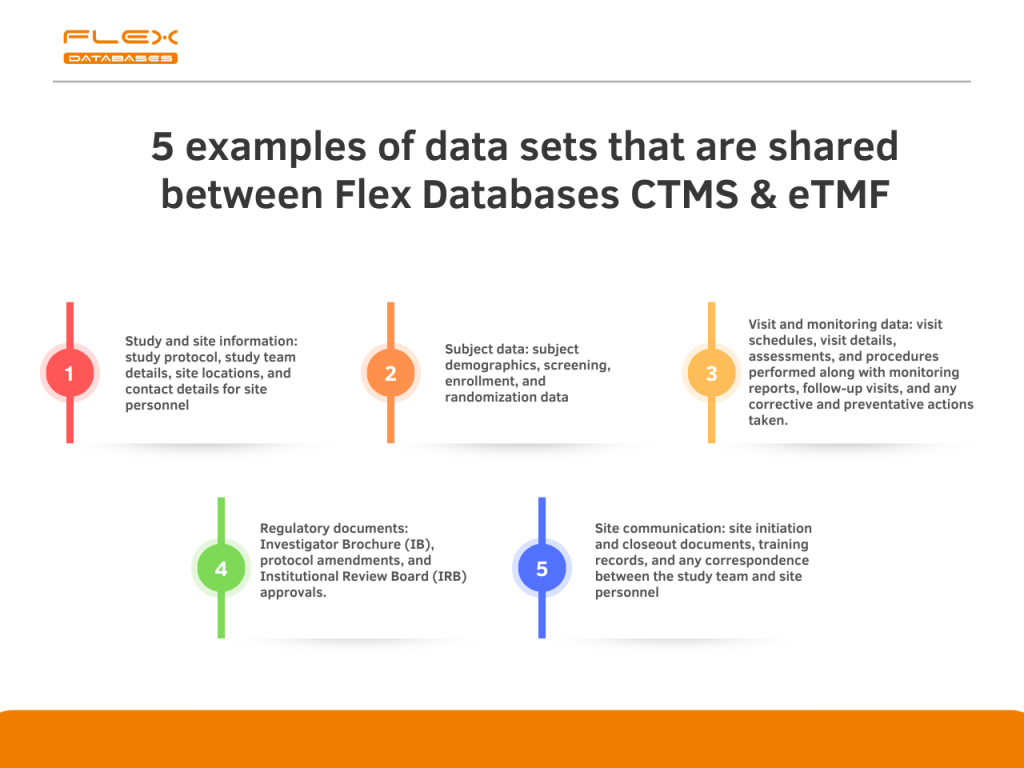
A study published in the Journal of Medical Systems found that integrating eTMF and CTMS systems resulted in a 50% reduction in the time required to identify missing documents and a 75% reduction in the time required to resolve missing document issues. Flex Databases CTMS & eTMF are interconnected to deliver you the highest possible […]
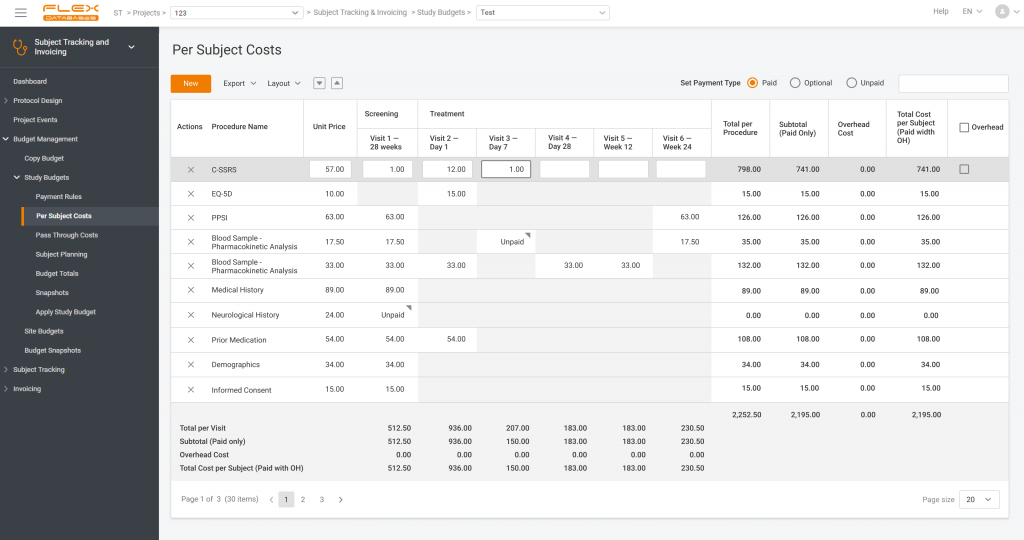
How long does paying to the sites while running a clinical trial take you? Average figures usually range from 4 to 6 months, even though in the contract, the payment period would typically be a month. Will you get frustrated if you are paid with a half-year delay? Yeah! Will that motivate you to work […]
“Why did you decide to implement CTMS?” is one of the first questions our CTMS clients get during a presale. We’ve collected top-5 answers for you to check and see if something hits close to home, and it’s time to start thinking about your own CTMS implementation. Here they are: And when it comes to […]
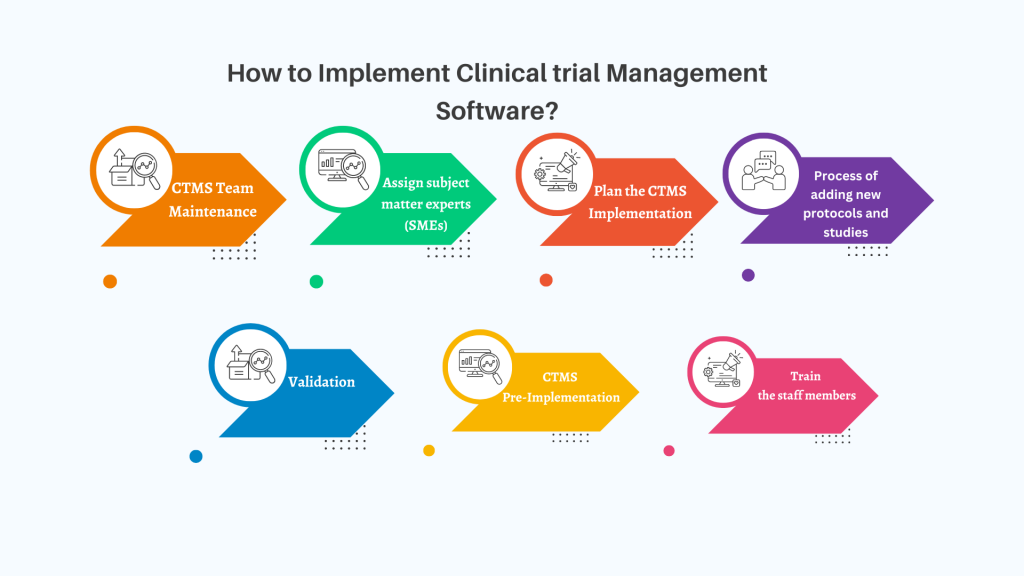
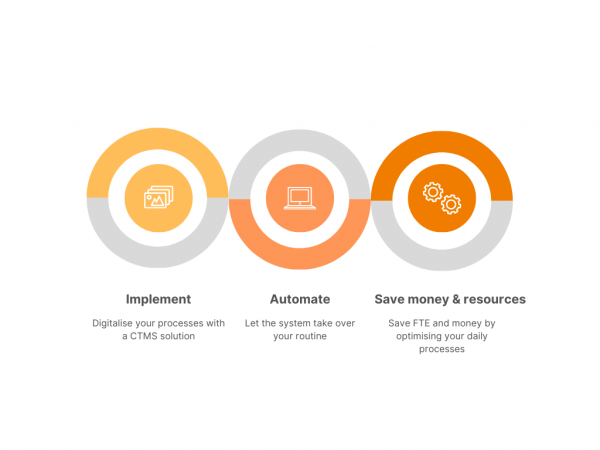
Clinical trials, as everything else in the world, is undergoing a heavy shift to process digitalization and automation. It has its ups and downs, but the future looks bright – we can minimize human error, optimize, and automate our processes and save money. Today we’ll talk about how exactly you can move from spending money […]
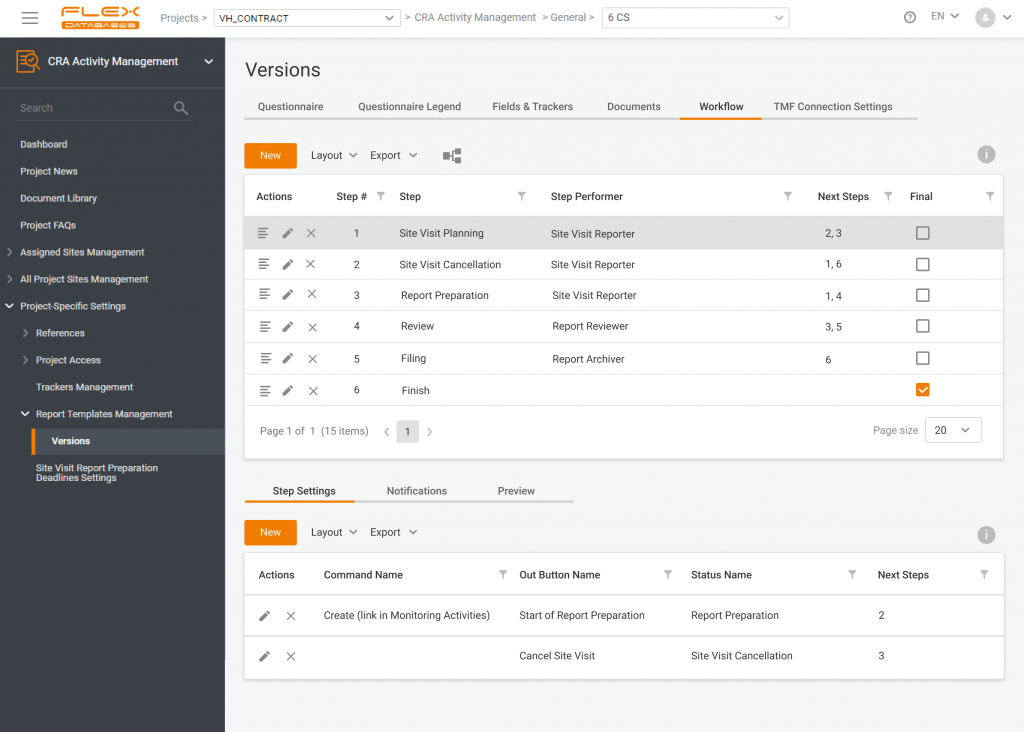
In clinical trials, any mistake can cost you a whole study. That’s why one of the essential things in the studies is processes and procedures. But what’s the first emotion you get about these words? Let me guess: disappointment, fear, irritation? It is probably connected to the fact that processes and procedures almost never work […]
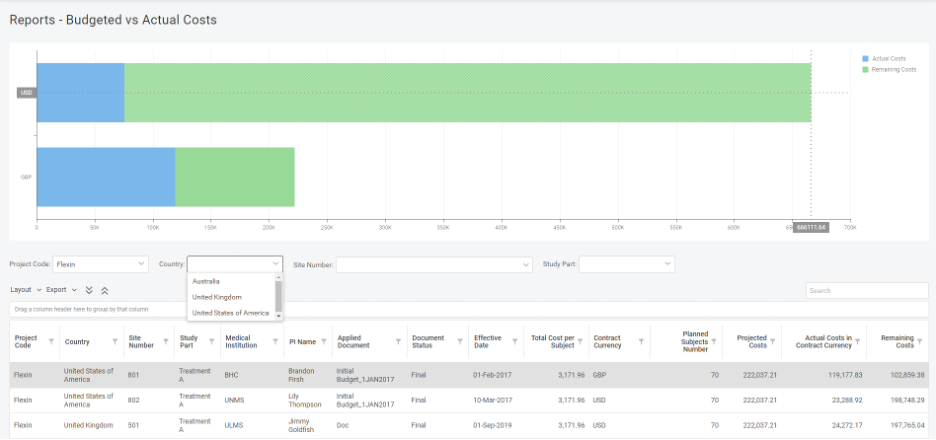
International clinical trials, in most cases, mean multiple currencies and exchange rates throughout all financial operations. Be it site or PI payments or transport and meal compensation for the patients; you will face the need to handle currency conversions in contracts and invoicing. Usually, everybody faces a lack of control and transparency when it comes […]
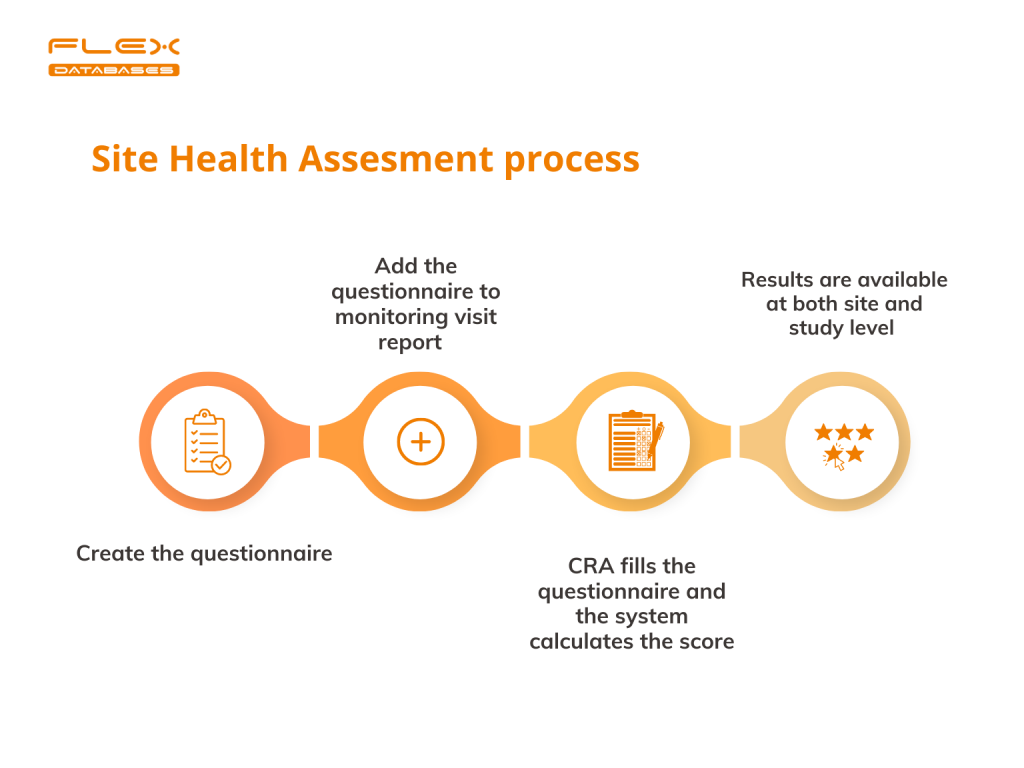
Risk-Based Monitoring (RBM) has become one of the trends in clinical trials of the last decade. Sponsors and regulatory authorities recognize RBM as an efficient approach to ensuring patient safety and data quality. We always keep track of market trends and develop features that facilitate our clients’ needs in implementing RBM techniques. What is Site […]
Clinical Trial Management System (CTMS) is an essential tool for any modern company, engaged in clinical trial process somehow. We’ve decided to compile an Ultimate Guide about Flex Databases CTMS and answer the biggest questions a person could have on the subject: from what is CTMS to what’s coming next. Table of contents: Chapter 1: […]
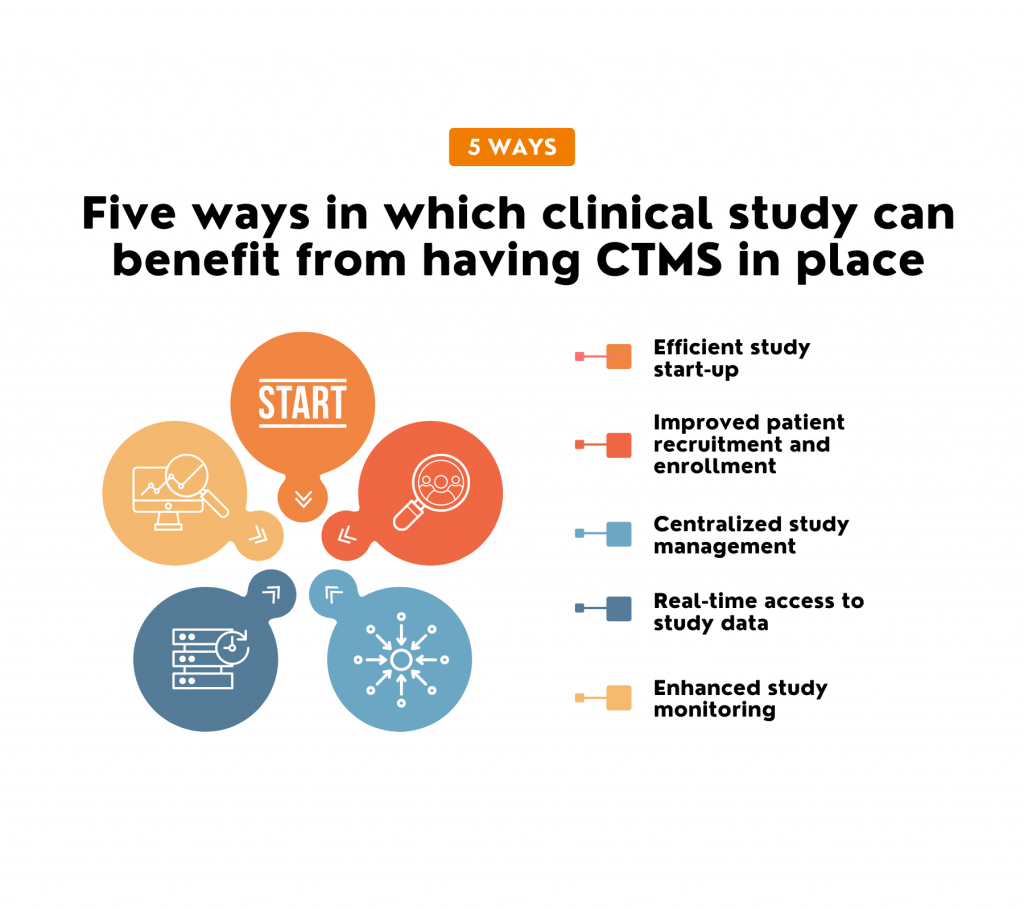
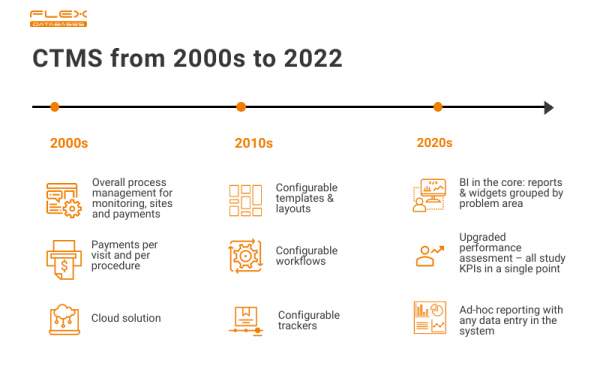
Insights on what makes the most impact on the project. Instant full picture in one interface. Sense of full control. Clinical Trial Management System (CTMS) is one of the most popular and widely used eClinical software applications. It’s been on the market for decades, and from simple process management, the concept grew in many different directions. […]
Digitalization, or moving to a new software provider for whatever reasons usually comes together with resistance. You don’t want to change your processes, since there are a lot of employees to get on board with the change. However, you still want transparency, performance analysis, and all those benefits of having an electronic system in place […]
In this video, we’ll tell you about Flex Databases CTMS & eTMF implementation for FGK CRO. We’ve reviewed the case study before, you can find the text version here. Timecodes:00:19 About Flex Databases00:46 VIdeo plan01:00 About FGK CRO01:44 Project Scope02:00 Project timeline: Preparation stage02:30 QA environment02:54 Production delivery03:15 Feedback from the client03:54 Contact us