Move from spending on FTE to saving with routine automation with Flex Databases CTMS
November 30, 2022
Clinical trials, as everything else in the world, is undergoing a heavy shift to process digitalization and automation. It has its ups and downs, but the future looks bright – we can minimize human error, optimize, and automate our processes and save money. Today we’ll talk about how exactly you can move from spending money on FTE to saving it with routine automation, brought to you by Flex Databases CTMS.
Here’s a graph from our Novo Nordisk case study:
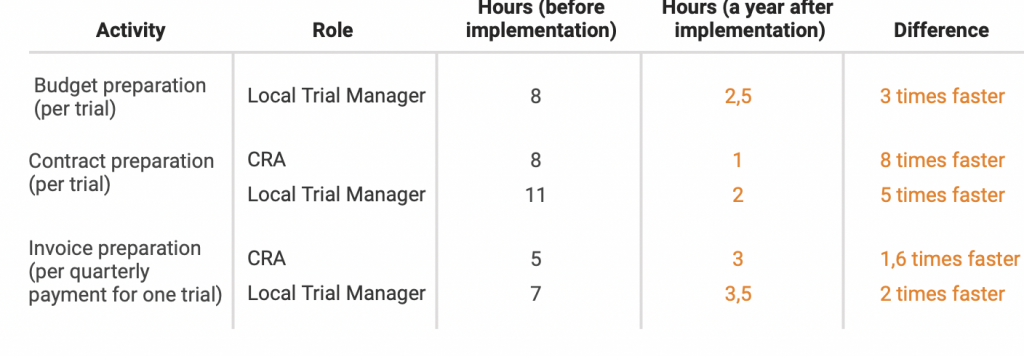
Let’s imagine, that our Local Trial Manager prepares 1 budget, 1 contract and 1 set of invoices per month to make our calculations simple.
Before CTMS implementation it would take them 8 + 11 + 7 = 26 hours in total
After CTMS implementation it would take them 2,5 + 2 + 3,5 = 8 hours in total
After CTMS implementation we’re saving 18 hours per month on budget & invoice management only.
Now we talk money. Salary.com provides us with $131,009 average annual salary for a Local Trial Manager in the US. With an 8 hour long workday, even without holidays is will bring us to the following rates:
Annual: $131,009
Monthly: $10,917
Hourly: $65
If we save 18 hours per month, it means that for an average Local Trial Manager we save $1170. ONLY on budget & invoice management. $14,035 per year.
Imagine, if we had two managers as an example for this calculation. Think about how many you have.
Digitalization is usually promoted as cost-effective – and here’s our take on that



