CRA Activity Management – your processes, but digital
December 9, 2021
Digitalization, or moving to a new software provider for whatever reasons usually comes together with resistance.
You don’t want to change your processes, since there are a lot of employees to get on board with the change. However, you still want transparency, performance analysis, and all those benefits of having an electronic system in place – to upgrade business processes and make data-driven decisions.
What if there is a way to do both – keep things the way they used to be yet go digital and get control over KPIs, overall transparency of the business and speed up processes?
Flex Databases CRA Activity Management delivers simple solutions by offering configurable workflows, templates and trackers. The system fits your processes like a second skin and provides you with a helicopter view to stay informed 24/7.
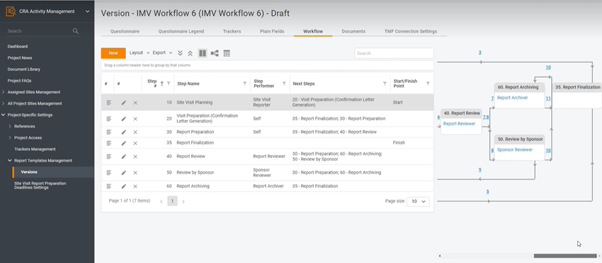
Here’s how it works: configurable tool means the ability to take a process, like Site Visit Report creation, and spin it however you like – set up new trackers, change or add steps, create documents templates, etc.
Let’s see an example: how can you add a new step to Site Visit workflow?

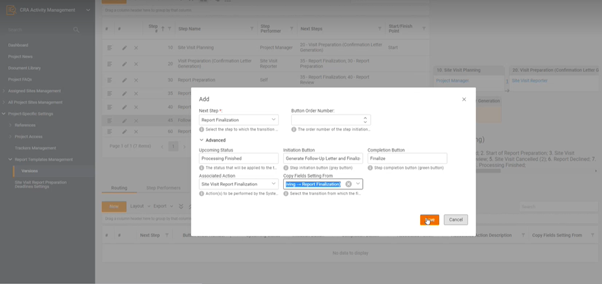
All you need to do is hit that “New” button and add the step:
- set up statuses,
- required actions like electronic signature or review,
- copy fields from existing step if needed,
- and choose the place that this new step will take in the workflow.
CRA Activity Management offers a wide range of possibilities together with being extremely configurable – for example, offline visit report preparation.
Are you afraid to ditch Excel or your old system, because it takes too long and too much, like an old habit? Don’t be. With Flex Databases CRA Activity Management we can make your processes digital – familiar, yet better.
Is it time to go digital and efficient now



