Use Case: Saving Time and Cost with AI in TMF Document Management
April 29, 2025
A mid-sized CRO managing 9,500 TMF documents annually faced increasing challenges with filing and metadata assignment. Each document required an average of 5–6 minutes to process manually.
The Problem
Manual document upload and metadata entry amounted to:
- 950 hours annually (based on 6 minutes per document)
- €47,500 in labor cost per year (at €50/hour average EU in-house rate)
This process also posed risks of human error and inconsistency.
The Solution
The CRO implemented an AI-powered eTMF solution with automated document classification and metadata fulfilment. The system reduced human involvement to minimal review and AI performance approval.
The Impact
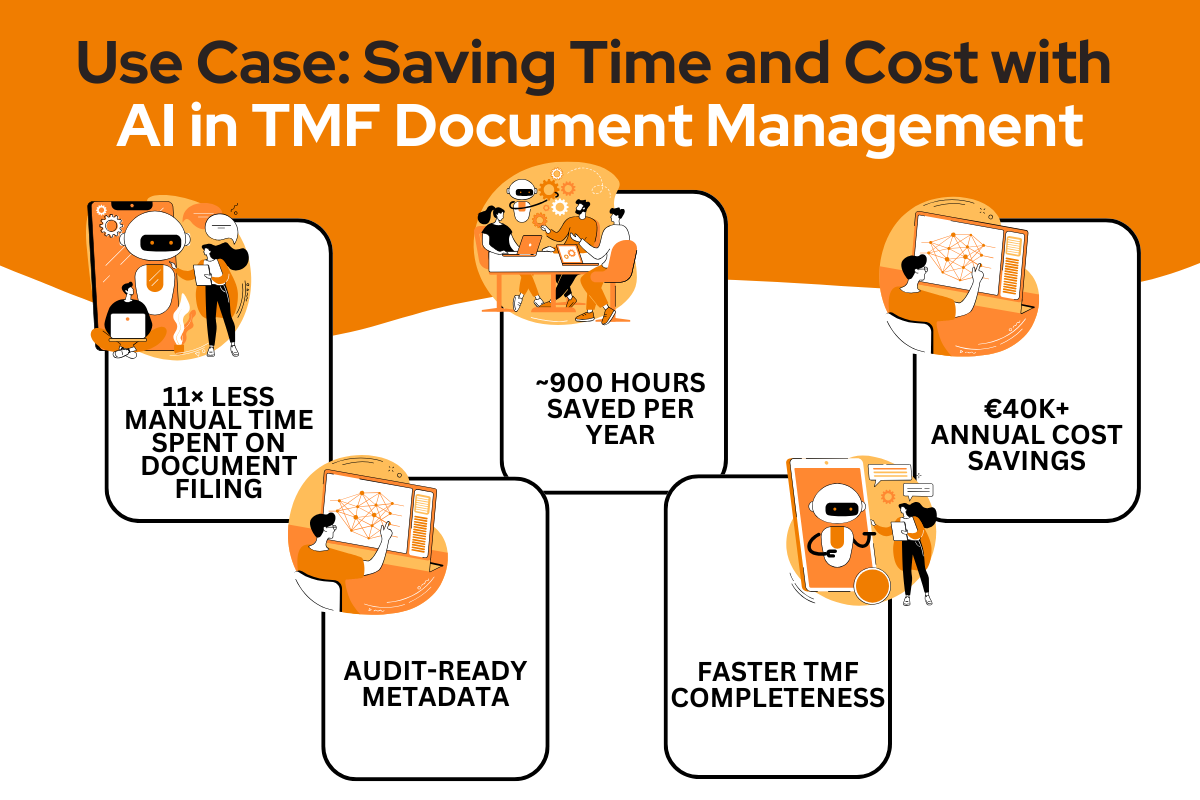
- 11 times reduction in manual time spent on document filing
- Estimated ~900 hours saved per year
- Annual cost savings of €40K+
- Improved compliance and consistency with audit-ready metadata
- Faster TMF completeness and quality tracking
Summary
By adopting AI in their TMF process, the CRO significantly improved operational efficiency, reduced costs, and increased confidence in their inspection-readiness. This case demonstrates how automation can turn TMF from a burden into a strategic asset.
| Metric | Before AI | After AI |
| Documents per year | 9,500 | 9,500 |
| Avg. time per document | 6 minutes | 0,5 minutes |
| Manual hours required | 950 | ~80 |
| Annual cost | €47,500 | ~€4,000 |
| Risk of human error | High | Minimal |
Looking to transform your TMF operations with AI? Let’s talk: contact@flexdatabases.com.



