Manage multi-lingual cases within a single instance with Flex Databases Pharmacovigilance
March 21, 2023
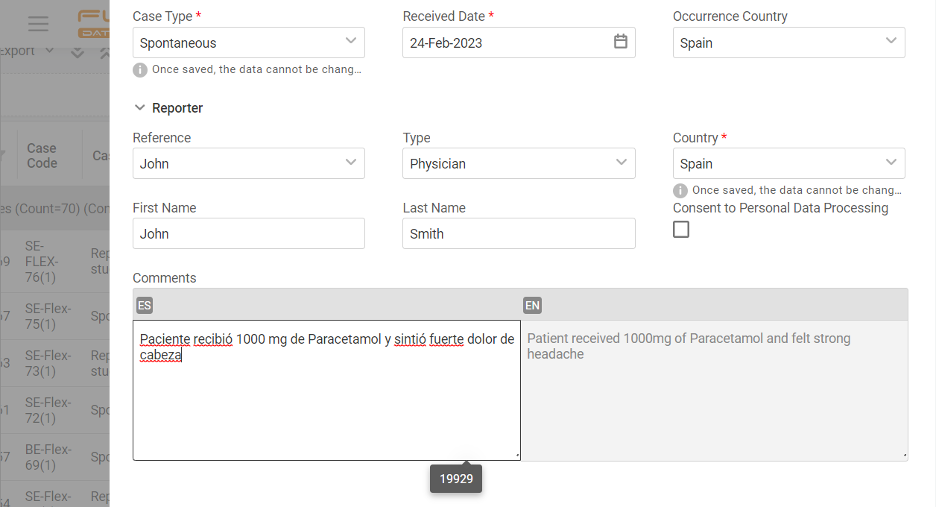
If you operate globally, you must comply with local language requirements, which also apply to pharmacovigilance reporting. Imagine that you must manage cases & report in English, Spanish, and French. How would you do that?
The apparent answer many providers usually offer is multiple inputs of the same cases. Drop that! With Flex Databases Pharmacovigilance, you’ll only need one case, and you can add as many languages to it as you want.
Look how simple it is.
Straight ahead when creating the case, type in all the needed information in all languages and avoid double entry from the first step of the process.
In addition, you can submit the reports to multiple agencies at once. Again, set the gateways to send the reports directly to the regulation agencies you want and proceed with as many submissions as you need. And keep the trail with a country submission report providing you with all the data, including the responsible employee. We have a case of submission to 40 countries in multiple different languages from our PV module, but the sky is the limit.
Is that something you would like to have? Reach out to our BD team to arrange a demo through bd@flexdatabases.com.



