Why Australia and Flex Databases are perfect for early phase clinical trials
April 16, 2021
When industry experts talk about the role of Australia in worldwide clinical trials, many mention its perfect fit for early phase trials conduction. Despite the noticeable remoteness, the country has many advantages to offer for clinical trial sponsors all over the world. Let’s outline the biggest of them and see, why Flex Databases is a perfect system for early phase clinical trial management.
- Cost-effectiveness
Australia: In 2011, the Australian government has launched the Research and Development (R&D) tax incentive, which has two components:
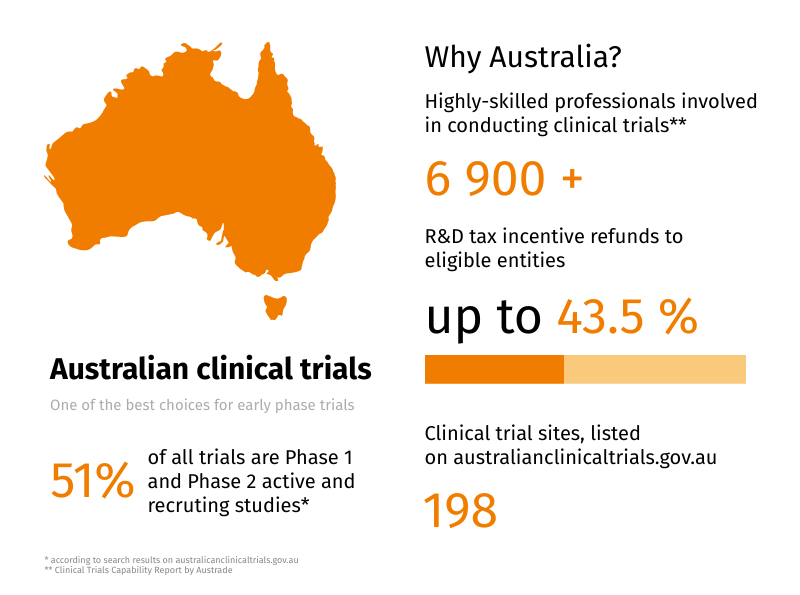
- a 43.5% refundable tax offset for eligible entities with an aggregated turnover of less than $20 million per annum, provided they are not controlled by income tax exempt entities
- a 38.5% non-refundable tax offset for all other eligible entities (entities may be able to carry forward unused offset amounts to future income years)
Basically, eligible entities can refund up to 43.5 cents per each dollar (AUD) spent on R&D. Sounds impressive, and we have only just started!
Flex Databases: We do not charge per project, so in one system for one cost you can have as many projects as you want.
- Simplified regulatory processes
Australia: According to Clinical Trials Capability Report, the majority of sponsored clinical trials in Australia are performed under the Clinical Trial Notification (CTN) scheme.
The CTN scheme reduces the number of assessing authorities to two:
- The Human Research Ethics Committee (HREC) reviews the documents and data, and also responsible for monitoring the conduct of the trial
- The institution or organization at which the trial will be conducted, referred to as the ‘Approving Authority’, gives the final approval for the conduct of the trial at the site, having due regard to advice from the HREC
Australian Therapeutic Goods Administration is only notified, and does not review the documents, yet still keeps the power to audit and enquire into the management of a clinical trial. Also, the ethics review process could be paralleled with site governance to shorten timelines even more.
Flex Databases: All our software is compliant with global and local Australian regulations and supports cross-country studies.
- High-qualified professionals, wide and developing clinical trials network
Australia: Over 200 000 professionals are involved in the Life Sciences industry in Australia, and over 6 900 of them are working with clinical trials. Australia has 37 clinical trial networks, 198 sites and more, than 50 independent research institutes.
Australian government is a big-time R&D supporter. In 2014 they’ve launched a 20$ billion Medical Research Future Fund, in 2015 – non-profit MTP Connect was launched as a part of the Australian Government’s A$250 million Industry Growth Centres Initiative, and the list goes on and on (remember the tax incentive?).
Flex Databases: We already work with some of the best Australian CROs and pharmaceutical companies.
In 2019, Yvonne Lungershausen, CEO at Avance Clinical explained, why they have picked Flex Databases software: “We found Flex Databases to be incredibly responsive, adaptable, they’ve listened to our issues, were proactive and logical in providing a solution for us. With the implementation of Flex Databases CTMS and eTMF, we are going to see improved efficiency and effectiveness, greater transparency. We are providing the fast study startup you see in Australia, and as a partner Flex Databases really came out on top in terms of being able to support that service offering. I think, together we can optimize both of our services – those of Avance and Flex Databases and have something really special to present to the clinical trial industry”.
If you are working in clinical trials in Australia or just planning to and looking for a software provider – look no more! Send us an e-mail to bd@flexdatabases.com or request a demo via the button on top of the page.



