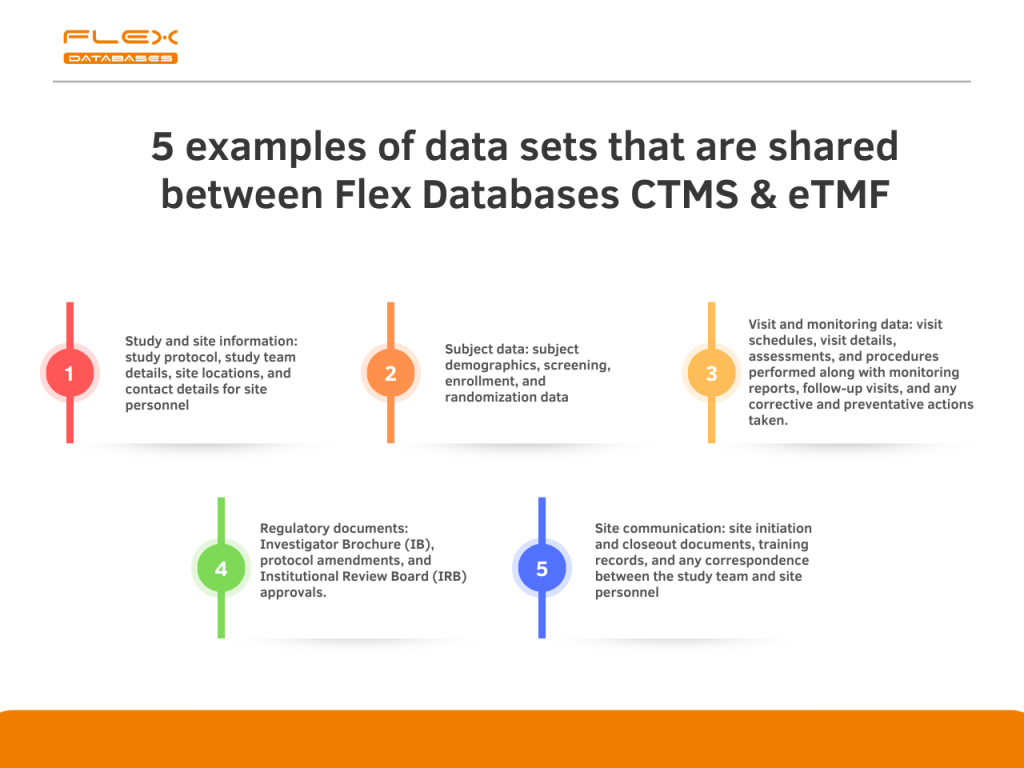
5 examples of data sets that are shared between Flex Databases CTMS & eTMF
May 19, 2023
A study published in the Journal of Medical Systems found that integrating eTMF and CTMS systems resulted in a 50% reduction in the time required to identify missing documents and a 75% reduction in the time required to resolve missing document issues. Flex Databases CTMS & eTMF are interconnected to deliver you the highest possible level of data completion and avoid missing documents issue.
Let’s have a look at the types of data that are shared between our CTMS & eTMF.
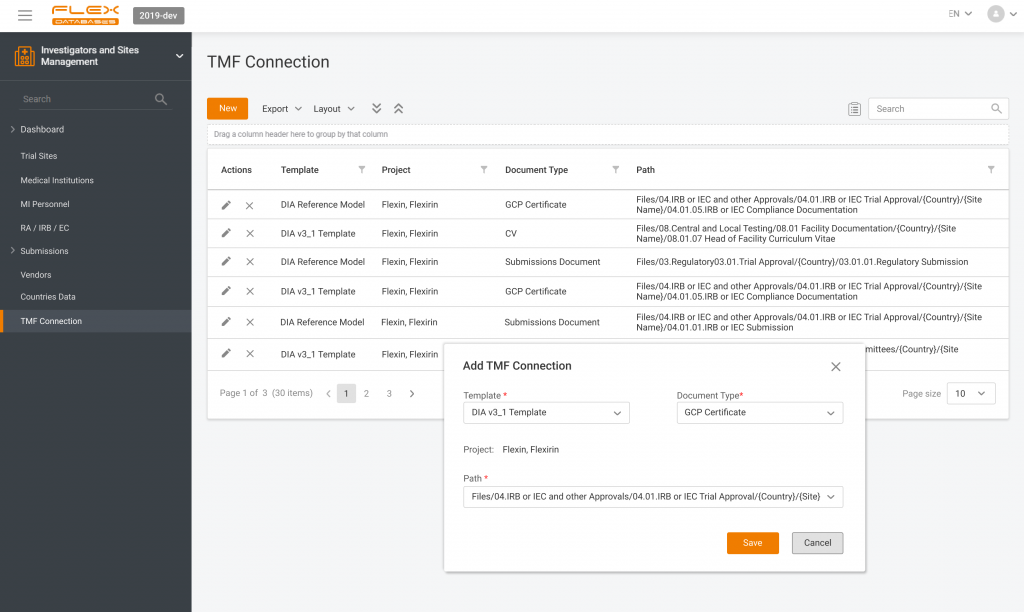
- Study and site information: study protocol, study team details, site locations, and contact details for site personnel.
Investigators and Sites Management – a part of CTMS where all the medical institutions are added once and then can be assigned as sites for any project
- Subject data: subject demographics, screening, enrollment, and randomization data.

Subject Tracking and Invoicing – a part of CTMS to track enrollment and distribute the information across the system
- Visit and monitoring data: visit schedules, visit details, assessments, and procedures performed along with monitoring reports, follow-up visits, and any corrective and preventative actions taken.
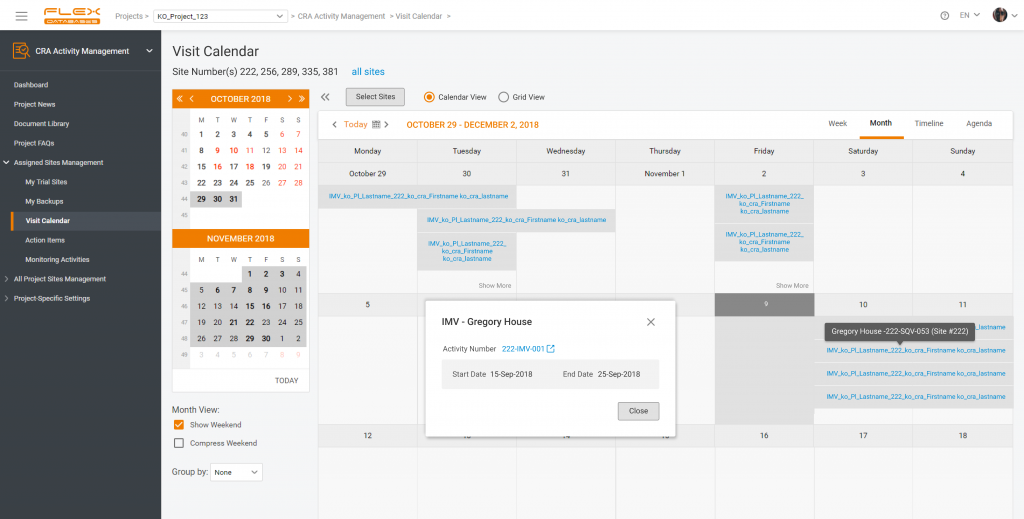
CRA Activity Management – a part of CTMS to arrange all monitoring activities, create monitoring reports, and manage the project
- Regulatory documents: Investigator Brochure (IB), protocol amendments, and Institutional Review Board (IRB) approvals.
- Site communication: site initiation and closeout documents, training records, and any correspondence between the study team and site personnel.
At the end of the day – an e-system is not a place to put all the information in, it is a place to find the information and use it. So go ahead and use everything you have in it. For example, once you created a monitoring report in CTMS send it directly to your eTMF and use it when needed.
Schedule a demo of our CTMS and eTMF to see how it works in real life!



