Answering top-6 questions about milestones
November 25, 2022
We kept six main questions in mind during the development of milestones. Do you want to know the answers?
- Milestones – why bother?
Milestones help us to:
- meet the deadlines,
- allocate time to each specific task of the trial,
- raise the visibility of the study in general.
Plus, milestones demonstrate the trial’s success and completion in achieving financial targets.
- Milestones in Project Catalogue – is the one-stop setup of milestones possible?
When you create a project in the system and enter its name, you can add the milestones. Doing that allows you to have the initial source of project milestones information in one place. That’s also the place where milestones automatically travel throughout the whole system from.
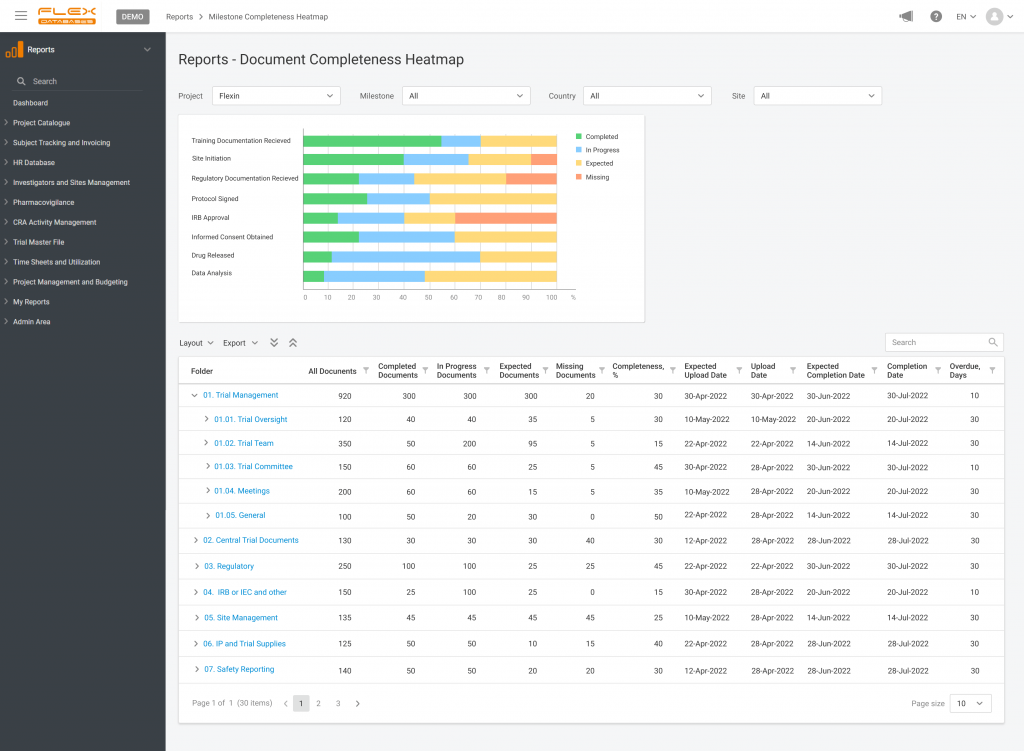
- eTMF completeness transparent as a day – are we there yet?
We are! Since the moment milestones are applied to your eTMF, the system will show you all the expected or missing documents by the milestone. Your team will know exactly what documents they must upload or finalize to meet deadlines and achieve specific milestones.
- Milestones in CTMS – can we finally see where we are and why?
Since milestones implementation, we achieved the utter level of synchronization between CTMS modules. If your PI enters the date of First Subject In at his side of the system – immediately this data will appear everywhere: in the CRAs’ part of the system, in the general project information, in the Project Management part, etc. Avoid delays by being aware of what’s going on in your project.
- Will my budgeted and performed now be automatically tracked and prepared for invoicing?
Take off the burden from both Project and Finance teams. When anybody enters the information on a milestone’s achievement in any part of the system – your finance team will see it on their part and can immediately proceed with invoicing.
- Milestones in Report Tool – I created something very specific yet again. Can I have a graph now?
Get a complete picture of what’s going on in your project. Use one of the inbuilt reports to assess overdue or upcoming milestones or go as crazy as you want – create any report based on the milestones in your system.
How to set up
How to track documents by milestones in eTMF
How to immediately inform all team members
How to automatically invoice by milestone
How to report in seconds
Do you want to learn more? Request a demo through the form or contact us at bd



