Blog Flex Databases
When working in a regulated industry, learning management is a huge concern since the need to follow SOPs & complete training applies to all company employees (at different levels, but still). It’s complicated enough when we think of dozens of people split between dozens of roles, but what if it comes to hundreds or thousands? […]
Do you remember when each new SOP or an update of an SOP required your QA and Training Teams to send out the emails to the employees? The days when everybody had to print and sign the acknowledgment forms? And then, the poor QA and Training Teams were to file the forms manually. It seems […]
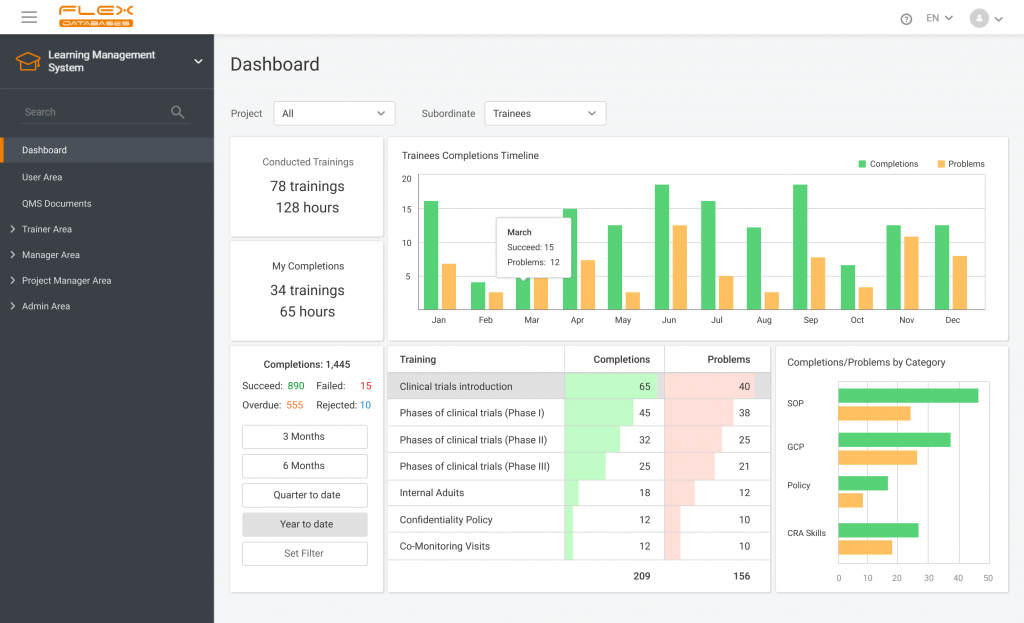
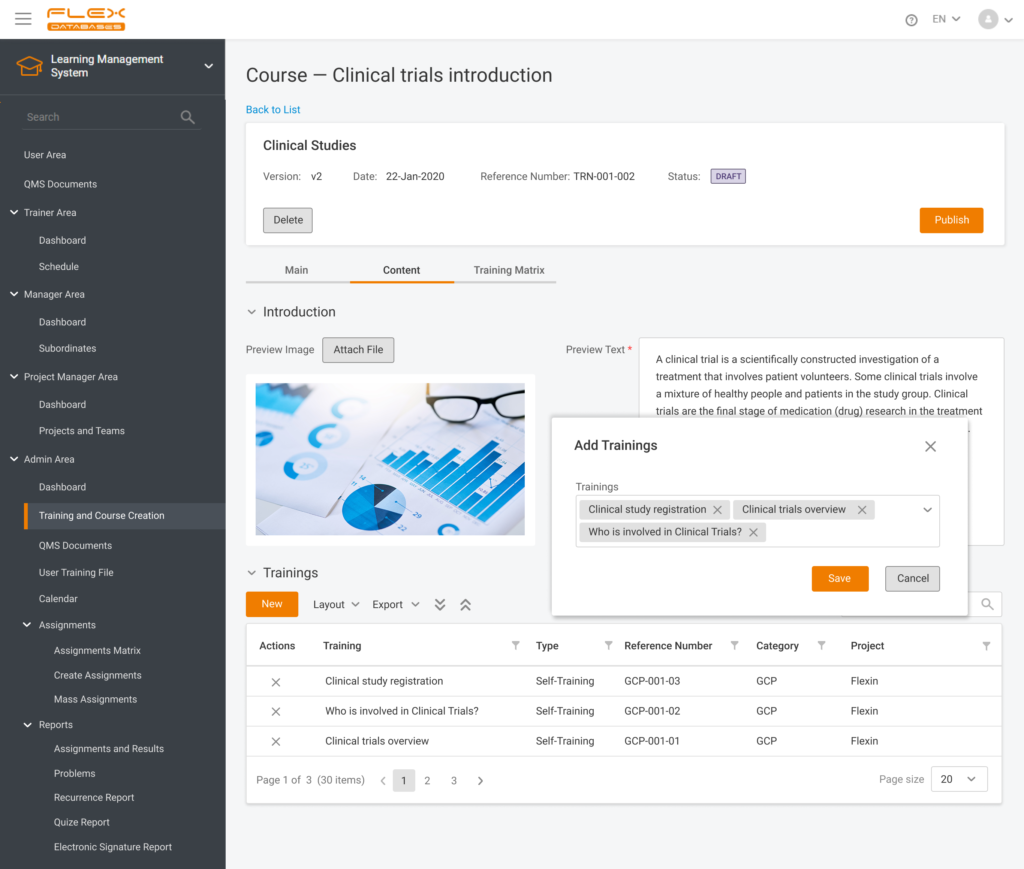
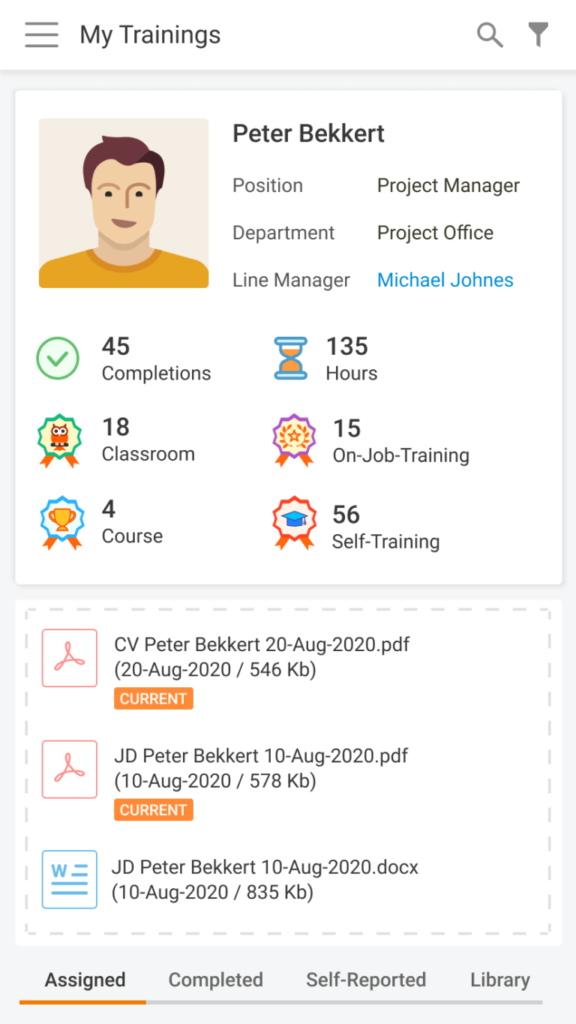
Nowadays, Learning Management System (LMS) acts as a virtual hub that helps not only with documentation, administration, reporting, tracking, delivery, and automation of training but mainly with audits and SOPs management. LMS has some crucial business benefits: There are tons of things to consider when it comes to choosing the right Learning Management System. Let’s take a […]
It’s been two years since our How to stop worrying and start working in clinical trials material dropped. Content-wise, it was the highest point for our Learning Management System so far. I have to admit, since then, we kind of abandoned its storytelling activities and just sometimes briefly mentioned new developments for LMS in our release notes. […]