Data integrity assessment according to ALCOA++: Flex Databases eTMF is fully compliant
May 20, 2022
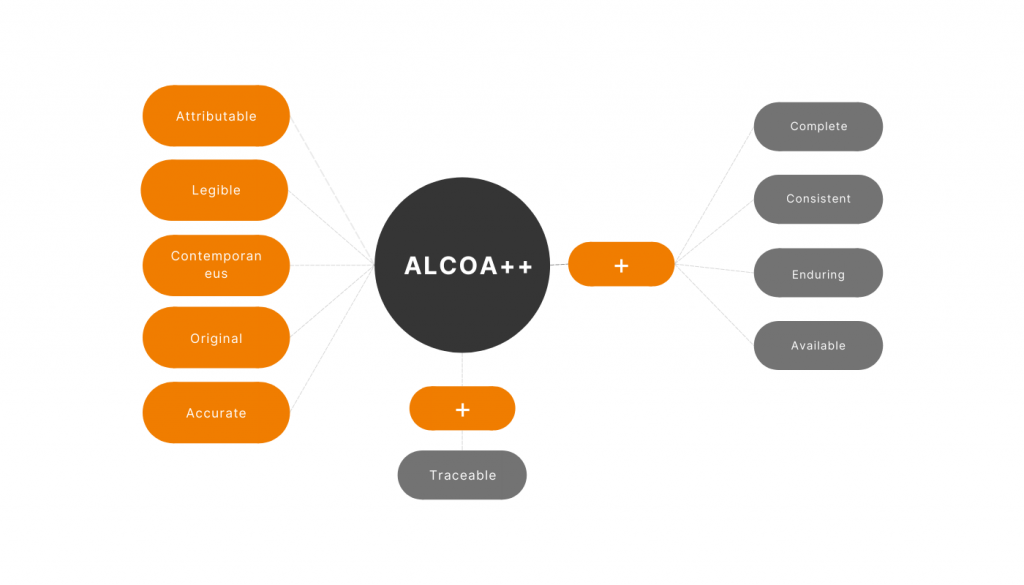
Data integrity has always been a critical point during the development of eTMF systems. One of the industry’s latest standards is ALCOA++, and our eTMF module is 100% compliant with ALCOA++.
Let’s see what advantages it brings to our clients.
A – Attributable
In our eTMF module, all actions conducted with each item are caught, kept, and indicated: who and when uploaded the file; all updates’ details are transparent and can be easily found right in the system. Extra attributes such as metadata can be used to mark documents for easy search or personal list creation.
L – Legible
Each item of eTMF has version history and actual information in easy-to-read format within the system.
C – Contemporaneous
All dates are available in the system’s interface. All the dates are indicated in one format in the whole eTMF module to avoid misunderstanding.
O – Original
The original records are permanently preserved in the system to save data meaning and integrity. Moreover, we can arrange a direct flow of the initial documents from sites into the system. In case of duplicates, the creator of the original record should confirm the authenticity of the copies.
A – Accurate
Our eTMF module allows its users to create amendments of any kind to the files, keeping a trail in the system. The version changes automatically, letting the users know all the actions taken to the file.
+
Complete
Placeholder-based visual statistics allow observing the eTMF completeness at any moment. If some previous or deleted versions are needed – you can reach them in the audit trail.
Consistent
As soon as an item is updated new version is tracked in the system automatically so that the content changes can be quickly investigated. When there comes an audit, you will be ready at no time.
Enduring
Stored data cannot be corrupted anyhow – all content changes are fixed at the log; any potential storage problems are covered by regular backups executed by the system automatically.
Available
eTMF web application allows the documents to be available 24/7 from any corner of the Earth.
+
Traceable
Timestamps with time zones specified allow eTMF to cover the last + of the ALCOA ++.
The reason for deletion provides an opportunity to trace even the necessity of action.
Reach out to our BD team at bd@flexdatabases.com to learn more about the eTMF module or to schedule a demo.



