Flex Databases eTMF – easy collaboration with an unlimited number of sites at no added cost
November 5, 2020
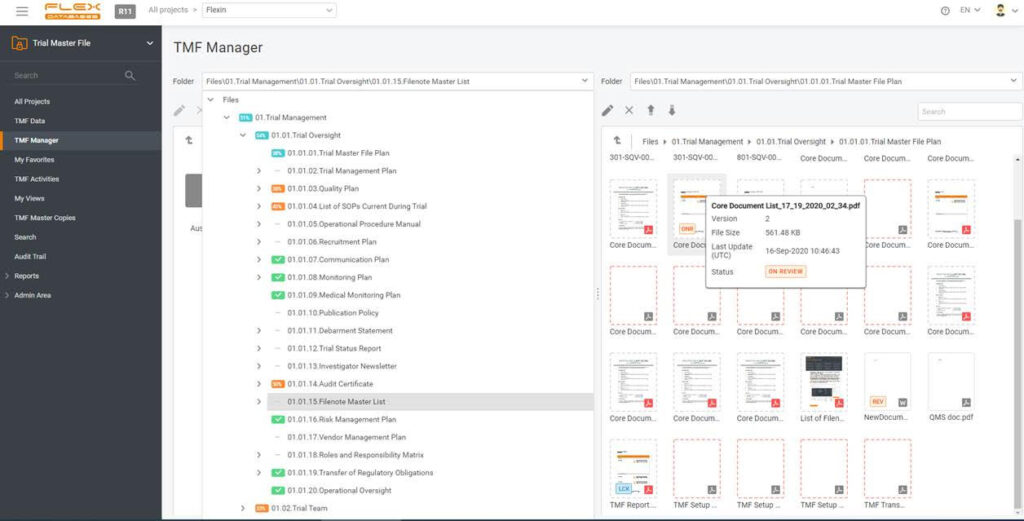
Documenting everything is a burden when running a study, especially a big and international one. Just think about putting all the documents in one place, and, most importantly, keeping this place tidy and structured. One must collect documents from many sites and can’t allow losing anything.
It usually costs a fortune to train site personnel and another fortune on top of increasing the number of users in some other TMF system. Well, not with Flex Databases eTMF.
In our case, sites simply email documents to the eTMF email address; documents are then available for Quality Check (QC) and allocation in a special folder. No added costs on the system, zero spending on training, and only one simple procedure to follow: email a document.
You can easily move documents to a correct folder with drag and drop function, assign metadata, and QC the document in one simple interface. No need to train sites in eTMF or provide them with access to your system.
Sounds interesting?
If you want to know more – request a demo via the button on top of the page, or send us an e-mail to bd@flexdatabases.com.



