eClinical solutions as a major means of inter companies’ communication in Clinical Trials
May 17, 2022
Are you the only one who needs access to the system?
In clinical trials, we often need to collaborate with a lot of partners for each trial or sometimes even to execute different tasks. And the more partners we have – the more difficult it appears to manage all the processes.
Moreover, many people think that adding some kind of eClinical solutions could only complicate the whole process, especially when it comes to the reality of every company using its own systems.
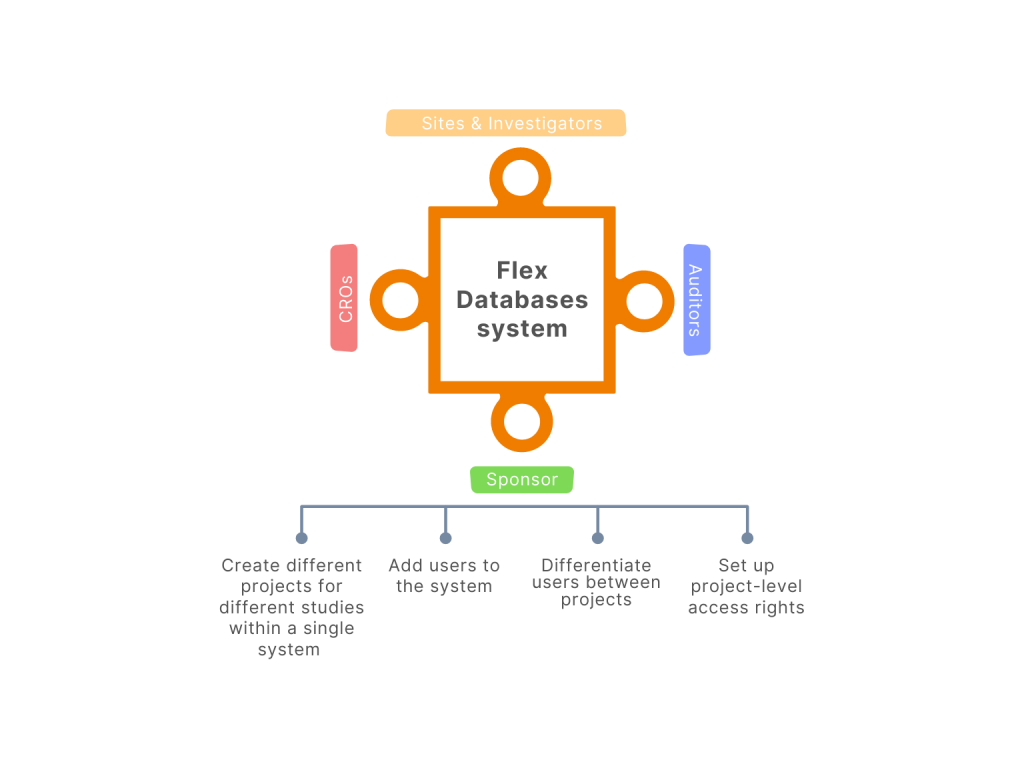
Flex Databases not just offers data flow from different partnering systems into ours, but we also do allow any users you want into Flex Databases systems.
How does that work?
Very simple. We provide you with a supportive one – HR Database – for free with every module. This module is created to add users to the system, store brief information about the users, and arrange accesses and notifications for the users. It means you are the one to decide who gets access to your system, be it your company’s employees or your partners.
Say you are a Sponsor using our eTMF system for your studies. You hire a CRO to run one of your trials, and you need several team members of this CRO to have access to the eTMF. To provide them with access, you simply add the users to the HR Database and indicate the rights you want them to have. Indeed, you can have multiple trials and different CROs conducting your studies. Will you want each user to see the information about all the studies? No way! And maybe you will not even want them to see some parts of the system within one project. And it is understandable. That’s why we developed our system the way that you can limit any access in the way you need it or blind parts of the system to exact users.
Another example:
You are a CRO, and you have multiple projects in your CTMS and would like to share some information with your Sponsors – again, you can do that by limiting each Sponsors’ users with the information you want them to see.
How will we charge for the users from your partnering companies?
Same as any other user you have in the system. Our pricing approach is based on the number of users. So, you will always pay only for the users you need to have in the system.
You can also need to give access to the system for your sites and Investigators. For instance, it would be a smart way to provide access to your sites’ staff and Investigators into the eTMF system. That will allow them to add all the documents straight into eTMF and not burden your team. But we do not want you to overpay for letting additional users from sites upload files. We created a new way: you will have an email address for the eTMF system where all the sites and Investigators can just send the files, not being the platform’s users, and the documents will be captured in the system. In this case, the files will be uploaded into your eTMF for free.
Reach out to our BD team at bd@flexdatabases.com to learn more about the advantages of our modules or fill up the form on top of the pa



