How to never miss a thing about your eTMF documents
January 13, 2022
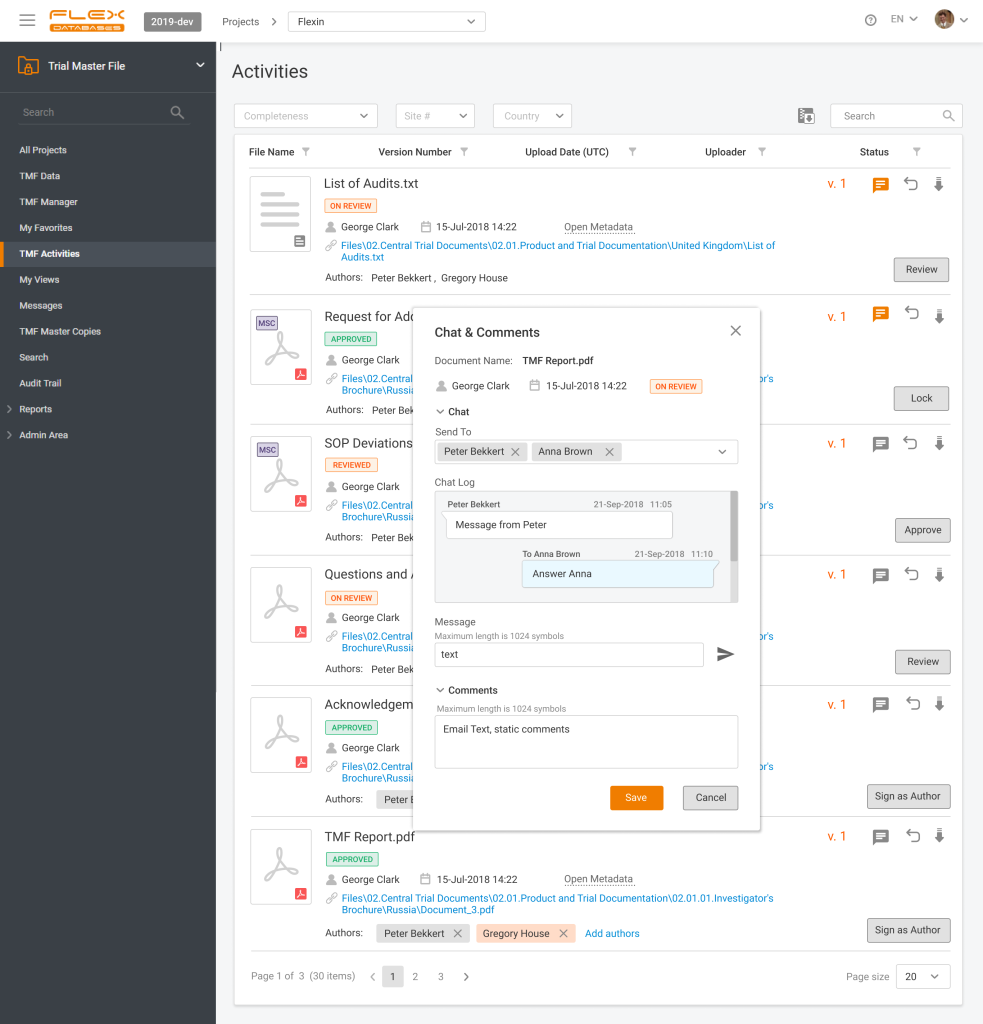
Have you ever missed a last-minute change in the file you’re working with? Do you lose track of thousands of messaging apps and emails? Do document review bugs you by being distributed into many channels, that you must align together?
It’s not a problem anymore!
With eTMF chat in Flex Databases eTMF everything, that you must know about changes/versions/updates in the document is here in one place. You’ll never miss a thing – if you have a message, regarding any document, all of it is right in the system and next to a document. You can easily see all documents queries, issues, and questions in a familiar chat interface easy to search and with instant access.

Do you have a comment on a document or a concern to raise? Send a chat message without long emails back and forth or extra explanations to what it regards to!
Instant source of truth about your eTMF documents – Flex Databases eTMF chat.
Request a demo now!


