How to adjust eTMF to your processes in 4 simple steps using flexible workflow
September 14, 2022
New software implementation is an imminent part of any digitalisation, but it does not come easy: out-of-the-box solutions are usually built with an average consumer in mind. It would tick the necessary and important boxes, but other than that you usually must adjust and shift your processes and procedures to it.
At Flex Databases, we aim to take you out of the vicious cycle by offering flexible workflow – to put it simply, a LEGO set of features that you can build however you like, to fit existing project requirements and SOPs, to adjust a system to you.
How it works:
With flexible workflow you get to be a designer of how things are.
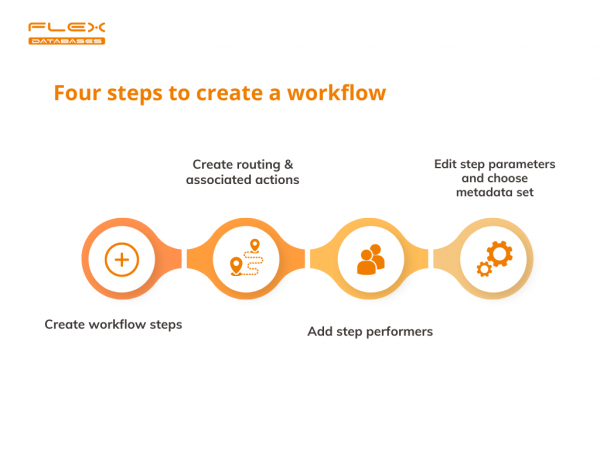
- First things first, create workflow steps:
Now we need to define created steps:
2. Create routing & associated actions, check required metadata:
3. Add step performers:
4. Edit step parameters and choose metadata set:
And you’re done, with the workflow that you’ve built the way you need it to be.
Possibilities are almost endless, but here’s a few examples of what you can do:
- Change the number of workflow steps to respond SOP requirements precisely to cover the SOPs
- Create and apply different workflows for different project tabs to make processes easier
- Assign parallel or sequential document review by the responsible users
- Make metadata editable throughout the QC workflow
We aim to provide you with the system that adjusts to you. Your processes, but digital.
Request a demo or contact us through bd@flexdatabases.com to learn more!



