How to set up eTMF quality checks in three simple steps
September 15, 2022
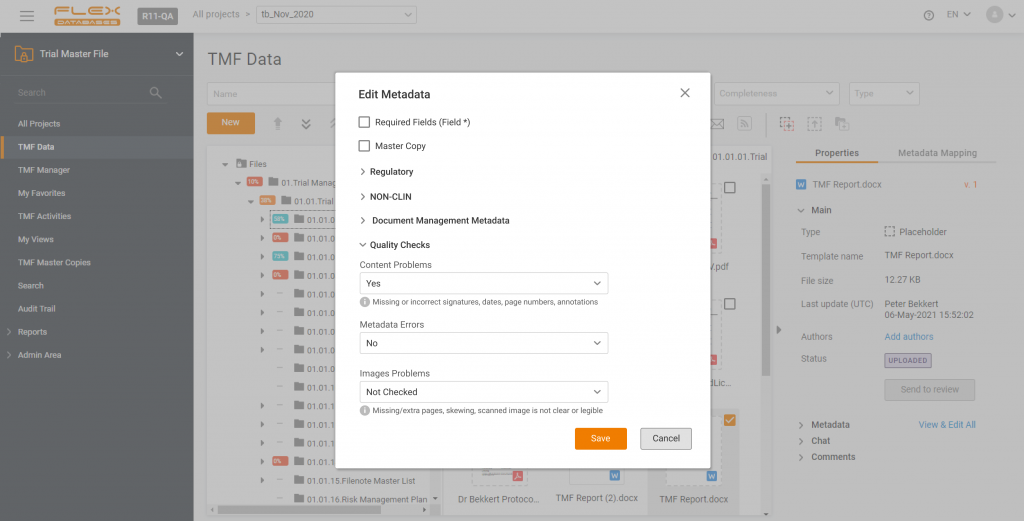
The difference between a paper Trial Master File and an electronic one is not only the storage and availability of documents. eTMF allows you to stay on top of your documentation in terms of quality – and here’s an instruction on how to set up Quality Checks in Flex Databases eTMF.
In Flex Databased eTMF, quality checks are part of metadata. We have a special metadata category called “Quality” (surprise!), and all metadata fields of this category have a dedicated report on a dashboard, so you’d get all the information about the quality of your documents at a glance.
Step 1: Create a new Metadata field
Step 2: Add the field you created in the project settings
Step 3: Check the new check field
And now you can see it in the report!
The quality check tool is flexible to cover all your needed SOPs’ requirements and team requests. Since any information can be presented as metadata, you decide on exact characteristics that should be checked in different parts of your TMF. You can predefine the quality checks for a specific placeholder, files, or even information resources, like users or sites – any filter is available in our eTMF.
Always stay on top of your quality with Flex Databases eTMF. Request a demo or contact us through bd@flexdatabases.com to learn more!



