How FGK CRO automated their clinical trials in 5 weeks
July 26, 2021

At the very beginning of 2021 Flex Databases and German FGK CRO signed a contract for CTMS and eTMF implementation.
About FGK
FGK CRO is a full service contract research organization offering a complete range of clinical development and consulting services to pharmaceutical, biotechnology and medical device companies. FGK approaches each project, whether large or small, with dedicated, highly motivated, small teams and a full commitment to achieving and even exceeding your objectives.
FGK Project Scope
Implementation of six Flex Databases modules Estimated timeframe: 8 weeks Actual delivery time: 5.5 weeks | Modules: eTMF CRA Activity Management Subject Tracking & Invoicing Investigators & Sites Management Project Catalogue HR Database |
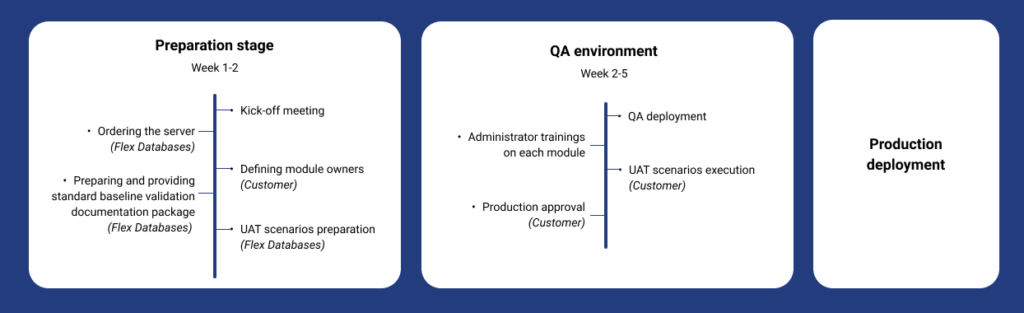
Three main stages of implementation
1) System deployment
- QA environment – for training and user acceptance with mock data
- Production environment – for real work and data
2) Training
- Online training session for administrators and end users
3) End user validation support
- Baseline validation documents for the system in general and per module to be installed, so that a customer could prepare according to their internal validation approach.
- Customer-specific documentation is issued by Flex Databases, for example, Installation Qualification including Maintenance Plan, UAT Scenarios, release documentation, and training certificates.
FGK implementation timeline
FGK feedback
 Martin Krauss, Managing Director of FGK Clinical Research | Why FGK decided to pick Flex Databases as a provider for CTMS and TMF modules? After an extensive evaluation, FGK chose the CTMS system from Flex Databases. The system convinced with its clear structure and intuitive handling. Both the flexible module for managing the payment of investigators and the possibility for CRAs to work offline with the system are clear advantages. Last but not least, the cooperation with the competent Flex Databases team during the evaluation phase also influenced the purchase decision. What do you expect to achieve with the implementation of Flex Databases? „FGK expects to standardize and simplify the management of clinical trials across different phases and sponsors by using the CTMS system from Flex Databases and thus to further increase cost-effectiveness and quality.“ |
Learn more about Flex Databases CTMS and eTMF or request a free demo: bd@flexdatabases.com.



