Flex Scan App
December 9, 2020
We keep making users’ lives easier! At least for early adopters.
Don’t you have your mobile phone on you all the time? It’s the only thing you need to upload scanned documents into our eTMF. No more broken scanners or bad connection – just take pictures on the go and spend your time on more important tasks. It also works for home workers – you no longer need to have a huge scanner taking place in your workspace.
How does it work:
- Put the document on a background that has high contrast, like a desk or table
- Press a button to scan a document in the app
- Adjust or crop if needed
- Convert to PDF or create a multi-page document if needed
- Upload created document to eTMF folder within the app
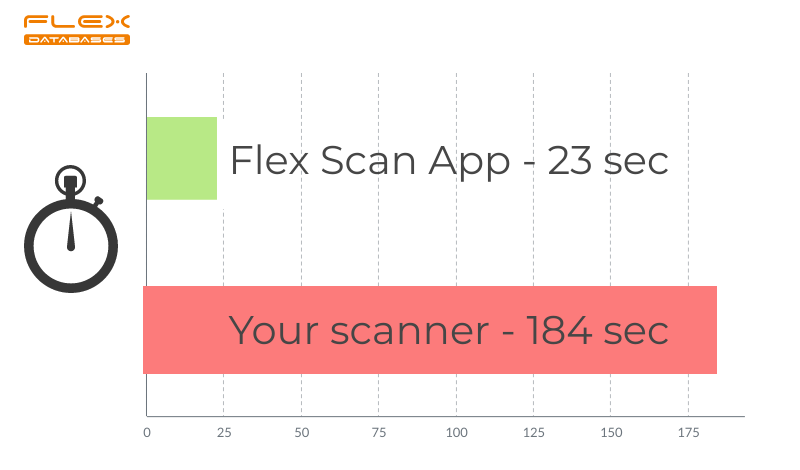
Here’s a comparison of the time spent on scanning with and without Flex Scan app. We’ve counted the average time our employees spend from putting a document into the scanner to uploading the scanned copy into eTMF – with Flex Scan app, it takes 8 times less time.
Benefits:
- Reduce time spent on scanning and uploading documents to eTMF
- Scan and edit documents from anywhere at any time, and the only thing you need is your phone.
- Edit scans you made with a few taps and choose only the important parts of the document to be transferred to eTMF
Wait no more, become our client, get in touch with us, send us an e-mail to bd@flexdatabases.com or request a demo via the button on top of the page!



