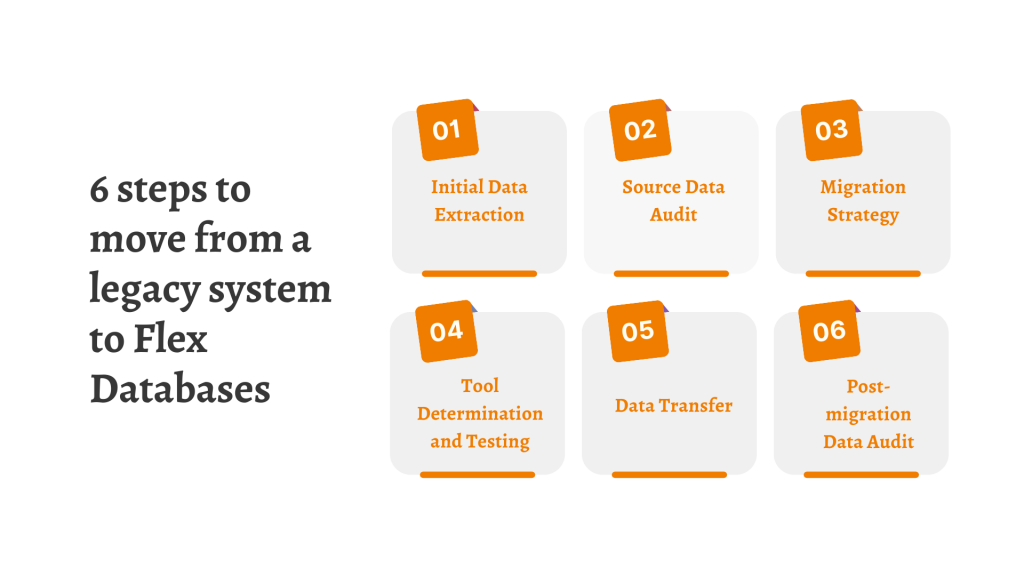
Move from a legacy system to Flex Databases in 6 simple steps
May 18, 2023
Digitalization is complicated not only the first time you go for it, but also when the moment for change comes. When we are a bit too dependant on a software provider, it may seem like there’s no way out, or data migration too much to handle – and it could be a blocker to consider a switch even if you are unhappy with what you have at the moment.
Here at Flex Databases we offer you a simple six-step approach to move all data from your legacy system to Flex, and we need your involvement only for three of those six. Here’s how it works:
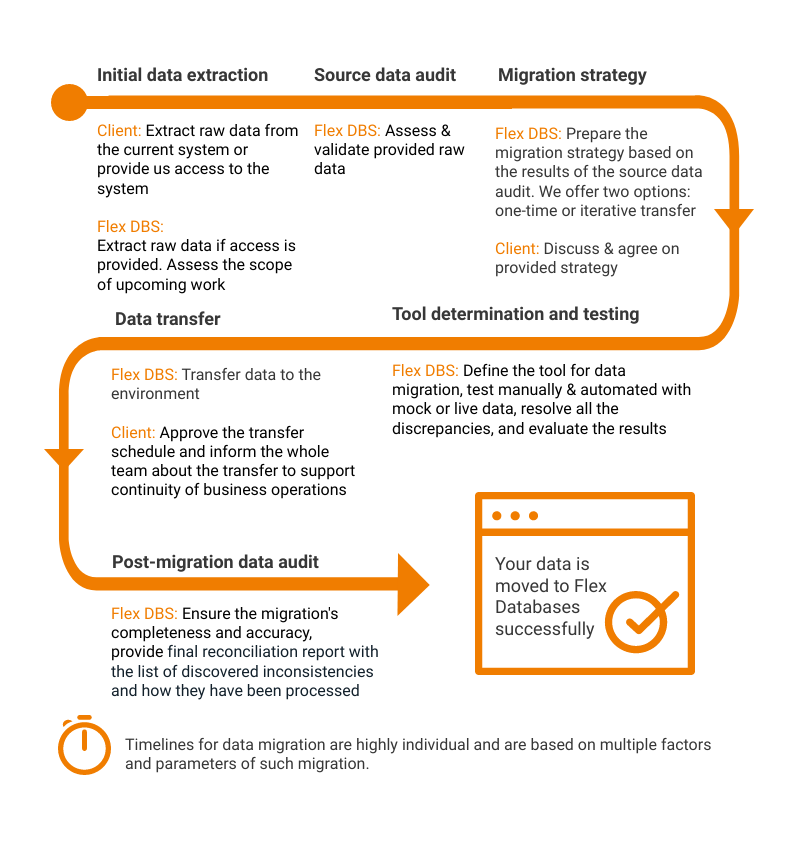
We also have a detailed guide on data migration, that you can find here.
If you are unhappy with the provider you have, or simply outgrown your current system, there’s no need to be afraid of changing it – we’ll be there for you



