How we implemented eTMF & developed SOPs in 3 weeks for iRenix
February 22, 2024
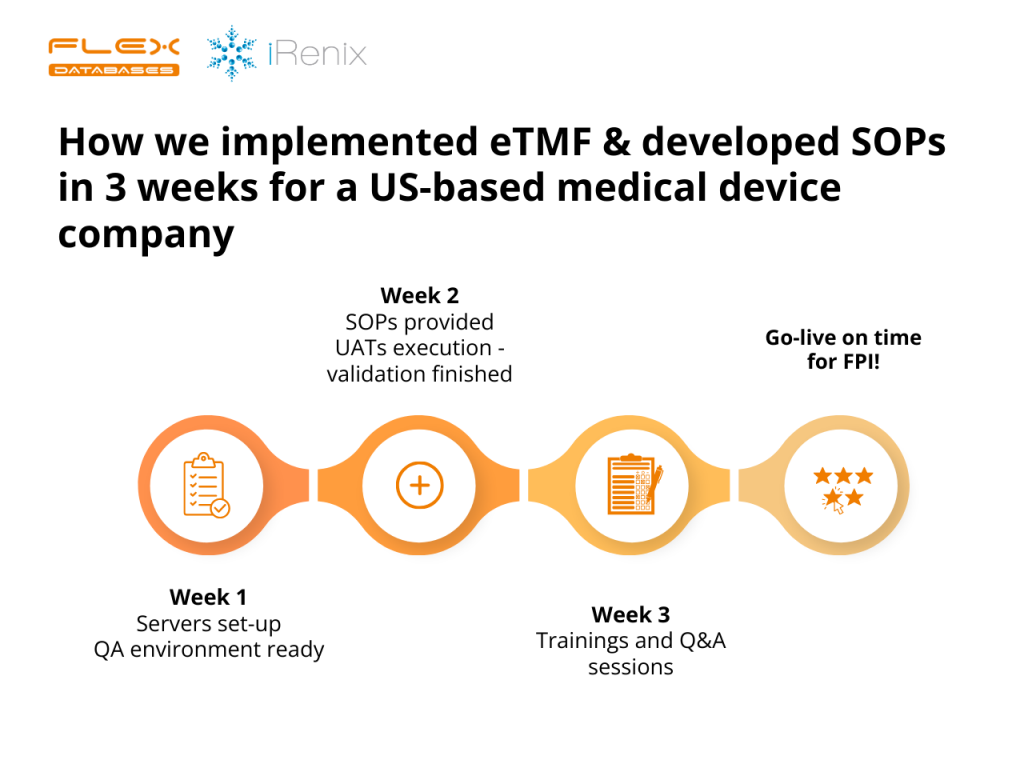
Last year, right around this time, we implemented an eTMF for a California-based Medical Device company, iRenix. Our target? A swift 3-week completion, so everything was up and running before their FPI. Now, this is quite the sprint compared to the usual 5-6 weeks!
And here’s the twist – while iRenix was laser-focused on their operations and study start-up processes, we rolled up our sleeves to deliver a full-on, turn-key eTMF setup from scratch. This wasn’t just about getting a system up and running. We crafted SOPs tailored for them to be included into their Quality Management System – SOP on electronic systems use, validation and eTMF, completed the validation process, and made sure the eTMF was ready to use. Our goal was to have the operations team simply log in and do what they do best in their clinical trials, without the headache of system implementation. They needed to start reaping the benefits ASAP, without being slowed down by the technicalities.
We turned iRenix CEO expectations into reality: “iRenix expects to have a seamless integration of this system into our clinical trial workflow, with excellent system support throughout the duration of our clinical trials”.
The system’s not just running; it’s thriving, on time, and with every piece of documentation in tip-top shape, approved, and organized. We’re in the business of making life simpler, not more complicated. We’re here to streamline, boost efficiency, ensure compliance, and yes – save time and money too!
Reach out and let’s chat about how we can make things a whole lot easier for you!



