Introducing Ideas Lab: a place where your ideas become our goals
October 28, 2020
Flex Databases system is well-known for its flexibility (you get it right from our name). We continuously develop our system and add new functionality. To create a roadmap, we follow clients’ requests to stay flexible, customer-driven, and relevant.
But where our clients’ ideas go? How do we make sure that nothing important is lost and we have it all on the table?
The answer is Ideas Lab.
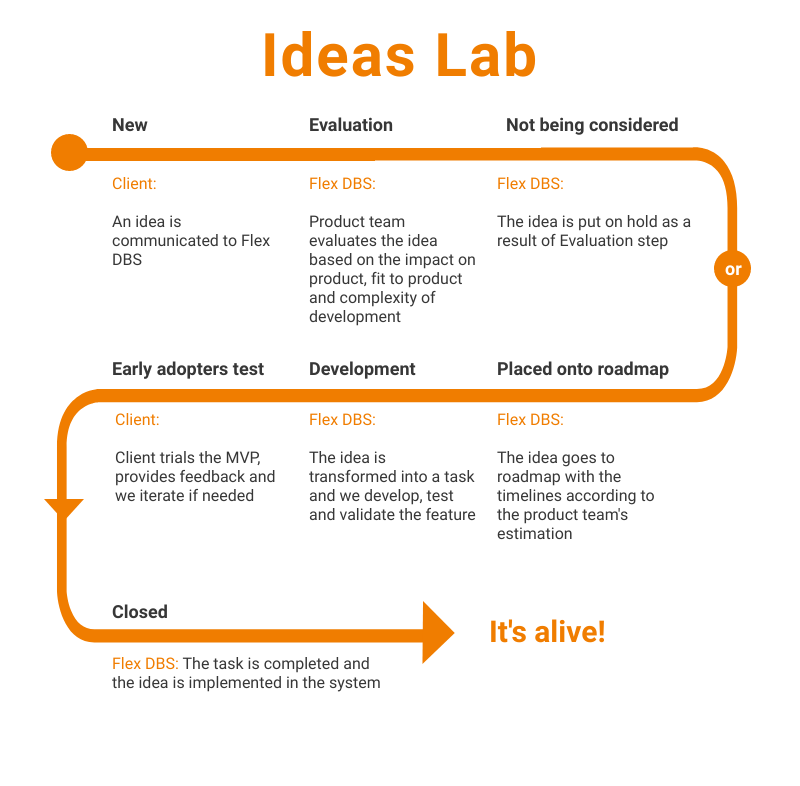
Ideas Lab is where we keep and process all of our customers – current and future – ideas. Every idea that we collect during the demo call, clients calls, or any other interaction is processed the following way:

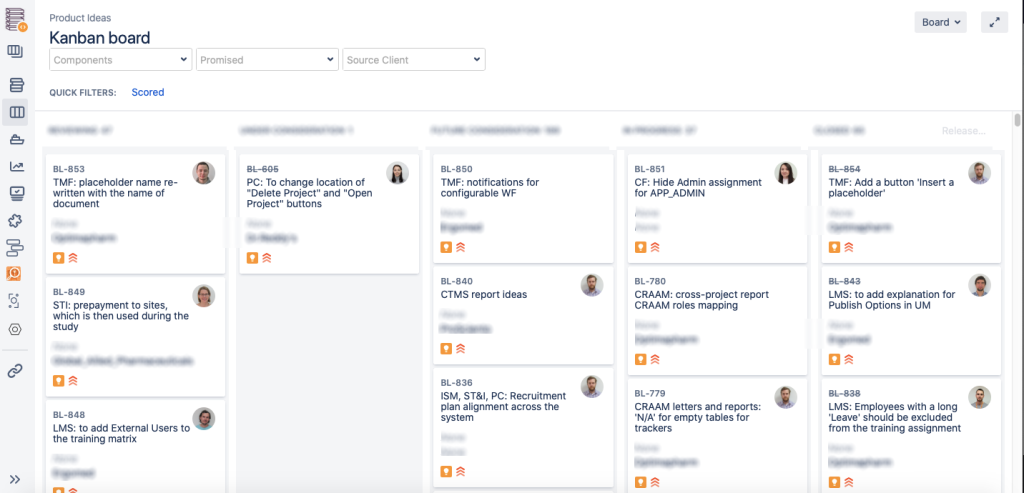
Here’s how it looks in real life:
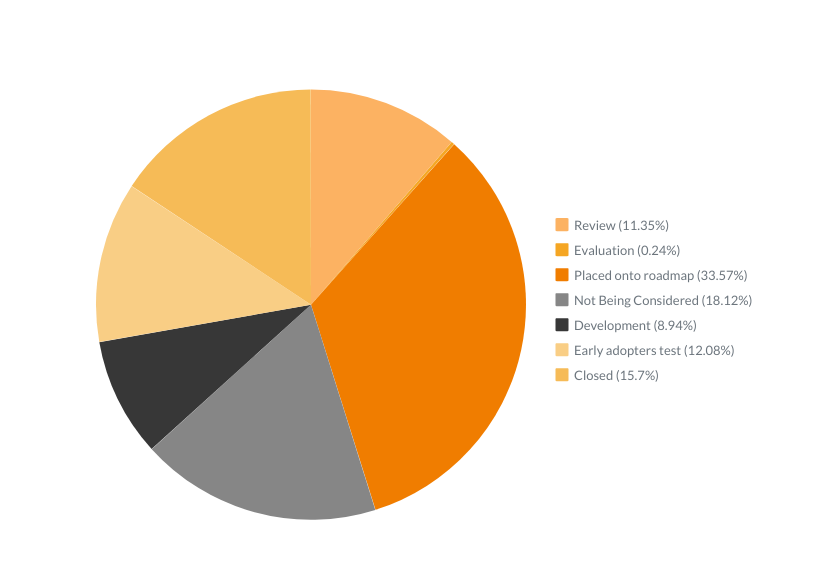
Out of 418 ideas we’ve received and recorded during the last three months:
- 47 are in Review
- 1 is in Evaluation
- 139 are Placed onto roadmap
- 37 are in Development
- 50 are on Early adopters’ test
- 65 are Closed
- And only 79 are Not Being Considered
If you want to share your ideas and become one of our clients – send us an e-mail to bd@flexdatabases.com or schedule a demo via the button on top of the page. We can’t wait to hear your ideas!



