Managing multiple-currency studies with Flex Databases
July 20, 2022
International clinical trials, in most cases, mean multiple currencies and exchange rates throughout all financial operations. Be it site or PI payments or transport and meal compensation for the patients; you will face the need to handle currency conversions in contracts and invoicing.
Usually, everybody faces a lack of control and transparency when it comes to multiple currency projects. Here are a few examples of issues that our CRO clients have faced:
Case 1. Except for the US sites, it is pretty standard for sites and investigators to get paid in their local currency as local regulations require. At the same time, all costs incurred by CRO are reimbursed in the currency, which is convenient for the Sponsor.
Case 2. Patient travel costs are sometimes paid in a local currency, and investigators are paid in EUR.
Case 3. Some activities performed by the site can be paid for in different currencies. For example, most patient visits are paid in EUR, but unscheduled visits require payment in local currency.
How Flex Databases platform can help you with multiple currency management
We have two financial modules which support the process:
- Subject Tracking & Invoicing for managing site and investigator payments
- Project Management & Budgeting for Sponsor-CRO service agreements and invoicing
Both modules allow incorporating multiple currencies into budgets and contracting terms. There are three ways to set up exchange rates:
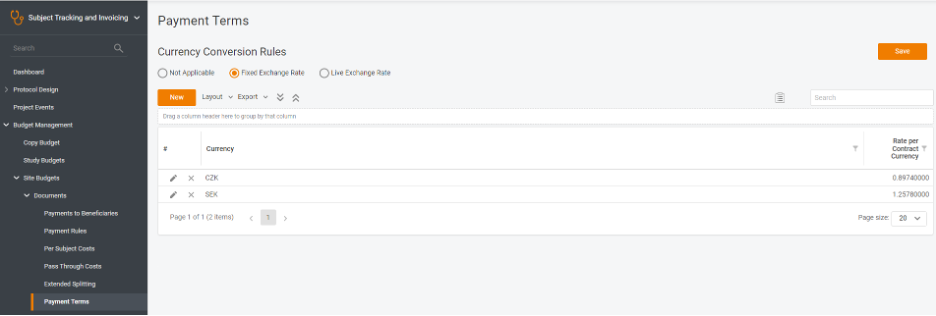
- Fixed exchange rates – in case your contracts have the baseline for the currencies, and they are strictly indicated, or you have them planned and continue applying the fixed approach to, say, forecast your budget expenditure and profits
- Online exchange rates – while generating invoices, the system can automatically apply the rates derived from oanda.com or any other source on the date of invoice or the date of payment
- Ad-hoc conversion – you are free of unbinding from the automated conversion and manually input any exchange rate to an invoice – the system will rely on that rate.
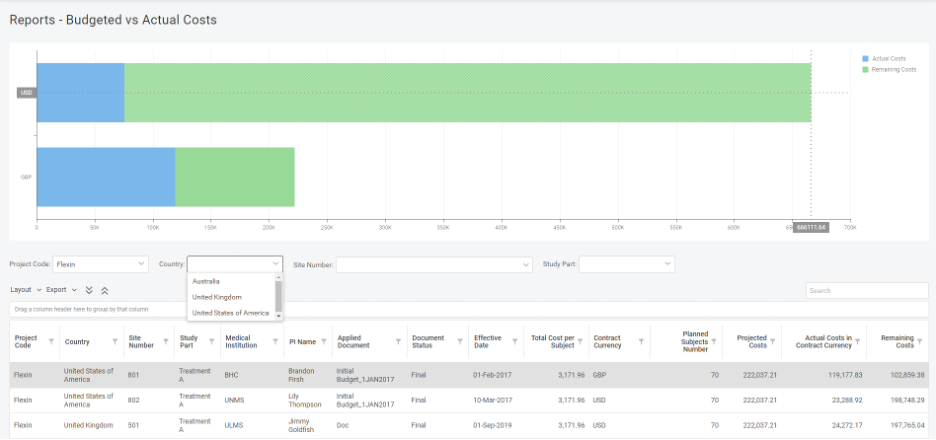
Aside from the configurable exchange rate, the system provides aggregate reporting in different currencies, which brings a lot of visibility and transparency to the entire process.

Stop struggling with the current rate issues and start using our convenient Project Management & Budgeting and Subject Tracking & Invoicing tools.
Sounds like something you’d like to try? Request a demo through the form on top of the page or contact us at bd@flexdatabases.com.



