How Novo Nordisk sped up payment process and cut costs with Flex Databases CTMS
December 19, 2019
About Novo Nordisk
Novo Nordisk is a global healthcare company, founded in 1923 and headquartered just outside Copenhagen, Denmark.
Their purpose is to drive change to defeat diabetes and other serious chronic diseases such as obesity, and rare blood and rare endocrine diseases.
Project background
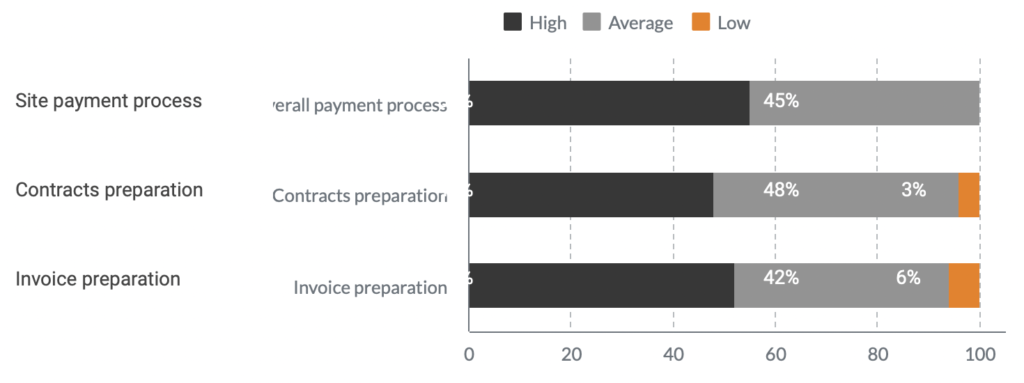
Most trial sites are working with no more than a 3-month operating budget, which means that maintaining up-to-date financial records is essential. Difficulties and delays in payment processes may cause a growing workload and burden, and this is why Novo Nordisk LLC came to Flex Databases to find a solution and fix the existing situation.

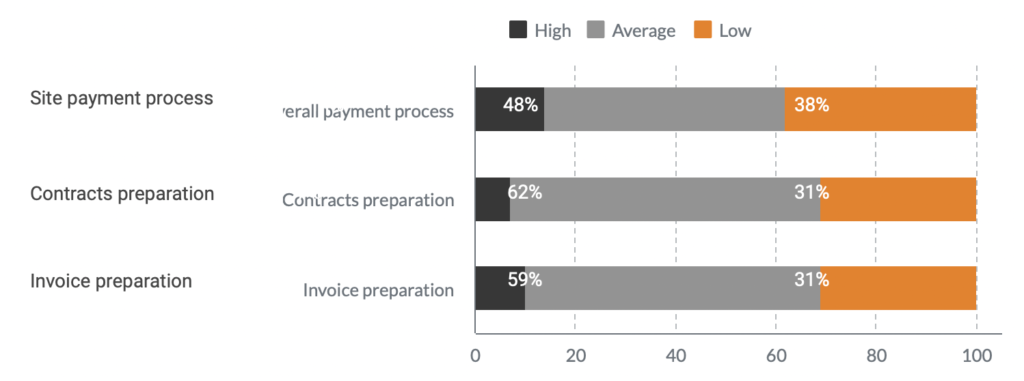
Workload before the implementation
Solution
Flex Databases offered Novo Nordisk to install a combination of CTMS modules – Subject Tracking & Invoicing and Investigators & Sites Management.
The accent was made on Subject Tracking & Invoicing since the module is tailored to proper full-stack grants management.
| Flex Databases Subjects&Payments | Flex Databases Sites |
|---|---|
| – All the information you need in one place: enrollment, inclusion curve, in and out of time window visits, screen failures and so much more. – Flexible and exportable widgets, reports, and graphs. – Site payments easily set, measured, organized, and tracked. | – Sites statuses, documents, contracts, qualification, vendors, timelines and so much more at hand. – All your Medical Institutions and Sites information in a clear organized manner with just a few clicks. – Submission packages across countries, studies, and sites in a unified interface. |
Result
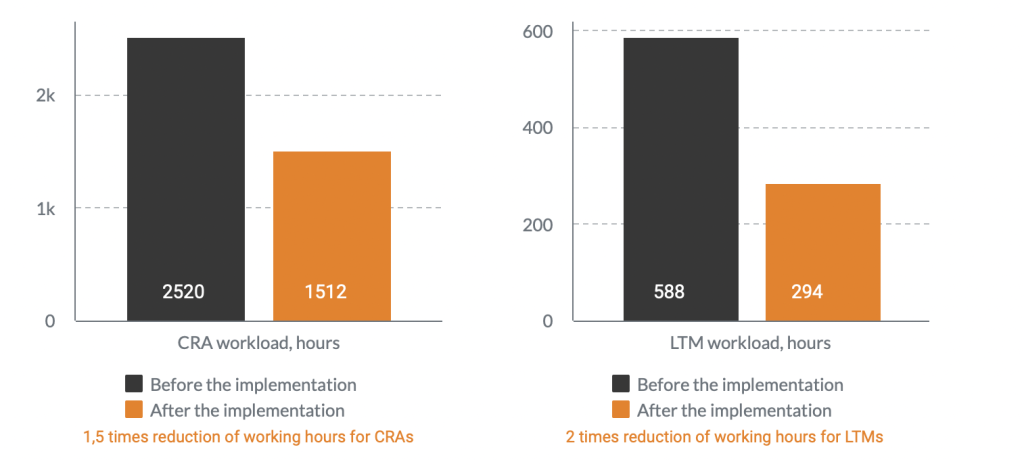
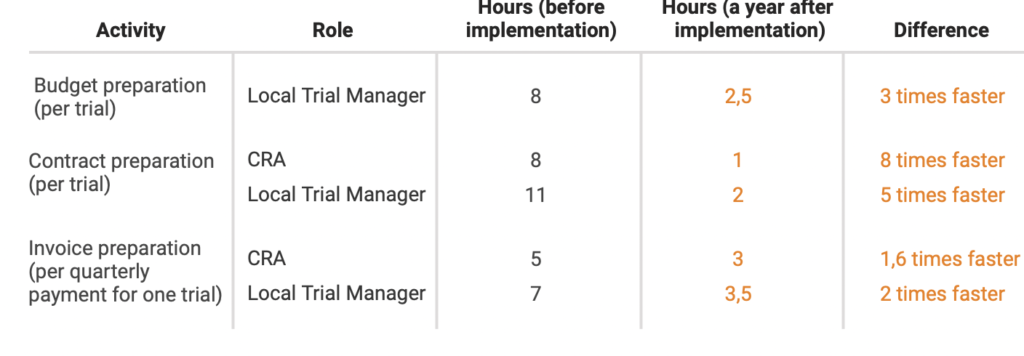
A year after Flex Databases CTMS implementation, Novo Nordisk sped up processes by 8 times and significantly improved overall process complexity management.
There was no need to hire additional staff anymore, the company has managed to allocate the available resources to different tasks and cut overall of payment processes.
Learn more about Flex Databases CTMS or request a free demo: bd@flexdatabases.com.



