Flex Databases Quality Management System – 1 system for 100% compliance
November 4, 2021
Quality Assurance is an essential part of any clinical research-related business. We built Flex Databases QMS module based on industry standards, latest trends, AND personal experience. A significant share of our employees is from QA departments of global CROs and have previous quality-related work experience.
Audit calendar & reporting
There’s a massive amount of internal and external audits to be scheduled and conducted during the year. And there’s a need to have a calendar. Before digitalization, we’ve marked the paper calendars or put it all in Excel. It is easy to imagine the problems: from sharing information with the team members to losing track and missing something important.
A simple yet powerful solution offered to you by Flex Databases QMS is a centralized audit calendar with automated notifications and action items tracking. You won’t forget something simply because you don’t need to remember it anymore – just log the audit to the calendar once, and the system will take care of reminding you whenever your attention is required.
What happens after happily ever after, aka when the audit is over? Reporting. We got your back here, too – all observations, tasks, and timelines are tracked automatically.
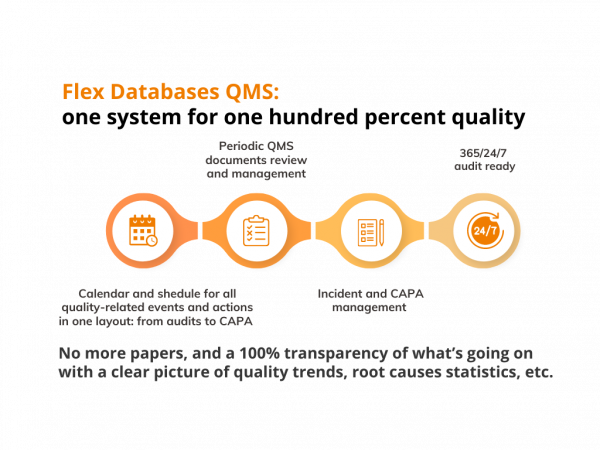
Calendar and schedule of events keep track of everything – audits, CAPAs, SOPs review cycles, etc.
Periodic SOP’s review
QA Managers must comply with the procedures periodic review schedule. Even if you have a document library, the human factor comes to action again – responsible for the review employees are usually busy, timelines for review and sign-off shift, and things may fall through the cracks.
Well, no more waiting for a response from reviewers for months – the system will take care of it and send you and a responsible person a notification with the reminder of things to be done. You also get full transparency on what SOP is on what step, who is the responsible person, how many days it took or how many days overdue, which helps to speed things up with a clear conscience. And there’s a sign-off and revision history log, so everyone who should be aware of changes in SOPs will get a notification.
Incident & CAPA Management
Regulations require incidents tracking. To keep it simple, Flex Databases QMS has an Incident Management Workflow – where any company’s employee can report an incident following the simple flow. After the incident is reported, you can grade it and involve a QA-related employee to oversee the process following the same flow. This way, you’ll have documented incident management evidence.
Even though not every incident leads to CAPA, any incident should be reported, analyzed, solved, and prevented from happening again in the future. However, IF there’s CAPA – you simply move along and initiate it within the same process, set up the responsible person, resolution, observation period, etc. Here’s an example of CAPA management flow:
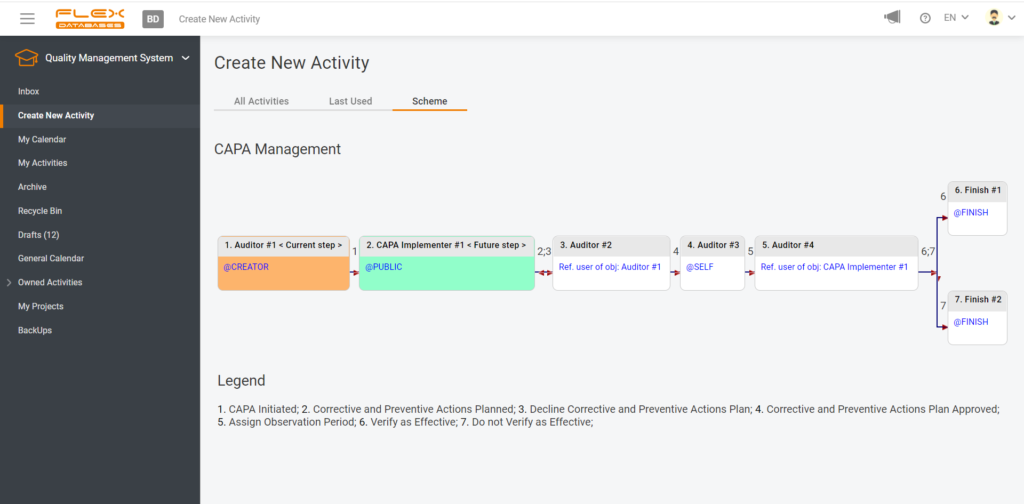
When it comes to CAPA Management, we must collect all internal and external CAPAs and keep up with the follow-up’s deadlines. The CAPA tracker sends automated notifications of upcoming deadlines and missing actions; shows you all pending and resolved activities, as well as all overdue tasks. And no human factor – you initiate a CAPA right after need occurred, and everyone responsible simply won’t be able to forget about it since the system will send reminders until all required actions are done. And, when it’s all set and done, a documented trail is here for you to present at audits.
No more papers, and a 100% transparency of what’s going on with a clear picture of quality trends, root causes statistics, etc. All quality data is here for you to stay 365/24/7 audit-ready, build a risk-based approach for your quality management, and ensure a hundred percent of quality with just one system.
Does it sound like something you need? Send us a demo request to bd@flexdatabases.com or simply fill out the form on our website.



