eTMF to arrange your Quality Review of Folder Completeness
February 11, 2025

It’s been out for some time now – Quality Review in eTMF of Flex Databases. But though we started with document review initially we expended this feature to folder completeness review capabilities.
Key Benefits of the Folder Completeness Review
- Track Folder Completeness: Monitor the status of folders throughout the review process.
- Assess Document Quality: Ensure reviewed documents meet quality standards.
- Verify Milestone Completion: Confirm that required milestones are fulfilled.
The Folder Completeness Review feature provides a structured and efficient approach to TMF quality management. By tracking completeness, ensuring quality, and verifying milestones, this update enhances compliance and oversight in clinical trial documentation.
Stay ahead in clinical trial management with our latest TMF improvements!
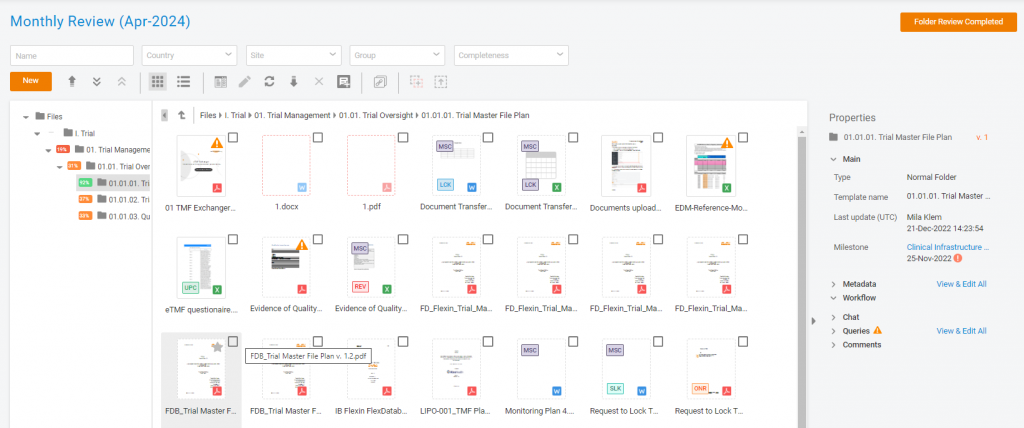
How to Use the Folder Completeness Review
- Initiate a New Review
- Navigate to Trial Master File → Quality Review.
- Click ‘New Quality Review’.
- Set the review conditions, select the target folders, and save the review.
- Start the Review
- Click the ‘Start’ icon to begin the review process.
- Perform Folder Quality Checks
- Open the ‘Review Card’ by clicking on the review name.
- Conduct necessary checks within a centralized interface.
- Access Documents for Review
- Click the ‘Open’ icon to view the list of documents in the selected folder.
- Complete the Folder Review
- Mark the folder review as completed by selecting ‘Folder Review Completed’ or checking the ‘Reviewed’ box in the Review Card.
- Finalize the Quality Review
- Click ‘Complete Review’ in the Review Card to confirm the review’s completion.