Flex Databases Application Release 10: What’s new
October 30, 2019
HR Database
- Ability to set up external users access and hide/unhide whole modules for different classes of users.
Investigators and Sites Management System
- Ability to use free text format in site number.
CRA Activity Management
- Offline capability: user now can fill in questionnaire offline and then upload it into the System
- Connection to the Trial Master File: the system automatically uploads documents (Confirmation Letter, Monitoring Report, Follow-up letter, Additional documents) with the correct naming right to the necessary folder in Trial Master File. The system will create a new folder for each visit automatically. Files will be already in Locked status in TMF, so there is no need to go through QC process for the documents twice.
- Configurable fields and trackers: user can now create any new trackers and fields in the system and add them to the Monitoring Visit workflow step. It’s possible to create several versions of a tracker.
- Configurable templates: user can now customize the layout of the system generated documents (Confirmation Letter, Monitoring Report, Follow-up letter), to add user-defined trackers, fields, tables and digital signature(s).
Subject Tracking and Invoicing
- Additional payment rules for Site payments: Complex Subject rule for custom subject payment, Holdback for open query for payment deduction in case of too many open queries, extra payment limitation.
- Multi-currency: different currencies for budgets and for invoices for different beneficiaries. Users may either use fixed or live updated currency rates.
Trial Master File
- Configurable QC Workflow: administrators are now able to define new steps of the QC Workflow in addition to the standard set of steps (Review-Approve-Lock). For example, you may add extra approvers to your flow.
- Automatic upload and filing of documents via e-mail: user may now send files directly to a specified folder in TMF via e-mail. Administrators may restrict only authorized users to use this option.
- Electronic Signature: user can now use User name/Password combination to confirm his approval/review of the document during the QC process.
- Archiving: administrator can create an archive of the project documentation or an archive of the selected folders including with Audit Trail and Index report.
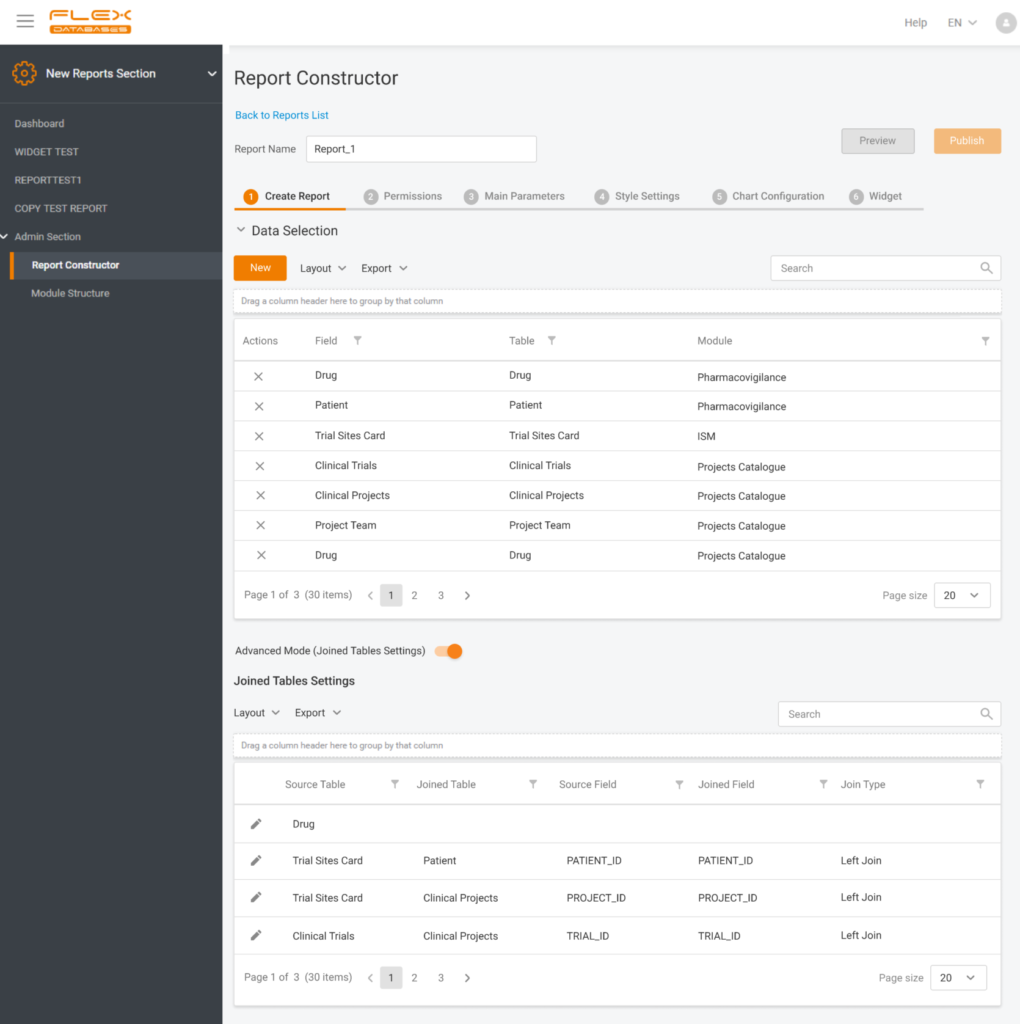
Reporting Tool
- Users can now create any report (pivot, grid), set up rights on position, project or site level, set up graphs and diagrams, create reports, using data across modules or projects.



