Revolutionizing Document Management: How AI Transforms Trial Master Files
June 18, 2024
In today’s busy world of clinical research, managing documents efficiently is crucial. Imagine being able to organize hundreds of documents in just minutes. Here’s how Flex Databases’ AI-powered Trial Master File (TMF) system makes this possible.
From Chaos to Order in a Few Clicks
The process is remarkably simple. Users can drag and drop a multitude of documents into the TMF. Within moments, while you’re grabbing a coffee or engaging in a quick chat, the AI kicks into action. It sorts each document, files them into the correct folders, and sorts metadata.
The AI Advantage
This transformation happens almost instantaneously. What once was a daunting pile of unsorted files is now a well-organized, fully compliant TMF. The AI ensures that every document is accurately filed and tagged, maintaining strict adherence to regulatory standards.
Unmatched Efficiency and Compliance
Flex Databases’ AI doesn’t just work fast, it works smart. The system operates continuously, processing documents as soon as they are uploaded. This real-time functionality means that as the volume of documents increases, the AI scales effortlessly, eliminating the need for additional staff and reducing operational costs.
Experience It Firsthand
Curious about how this revolutionary system works? Flex Databases offers real-time demos to showcase the AI’s capabilities. Witness firsthand how the AI transforms your document management process, freeing you to focus on more critical tasks.
For those ready to streamline their TMF management, book a demo on the Flex Databases website and see the future of document management in action.
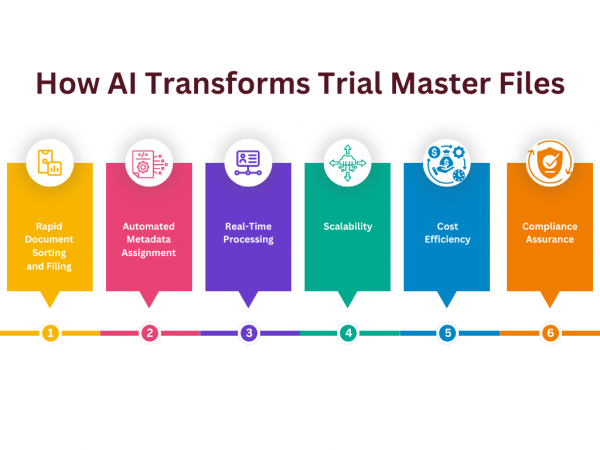
Key Features of AI in Managing a Trial Master File (TMF)
- Rapid Document Sorting and Filing: AI can process and organize large volumes of documents in minutes, significantly faster than manual handling.
- Automated Metadata Assignment: AI assigns the correct metadata to documents, ensuring they are filed accurately.
- Real-Time Processing: AI works continuously, processing documents as soon as they are uploaded.
- Scalability: As the volume of documents grows, AI scales up without the need for additional staff.
- Cost Efficiency: Automating the filing process reduces time, effort, and budget, leading to cost savings, particularly in large-scale studies.
- Compliance Assurance: AI ensures that documents are filed in the correct place with the correct metadata, maintaining compliance with regulatory standards.
Embrace the future of document management with Flex Databases’ AI-powered TMF system and experience unparalleled efficiency and compliance.



