RWE-ready eTMF for 24/7 transparency in your documents
June 14, 2023
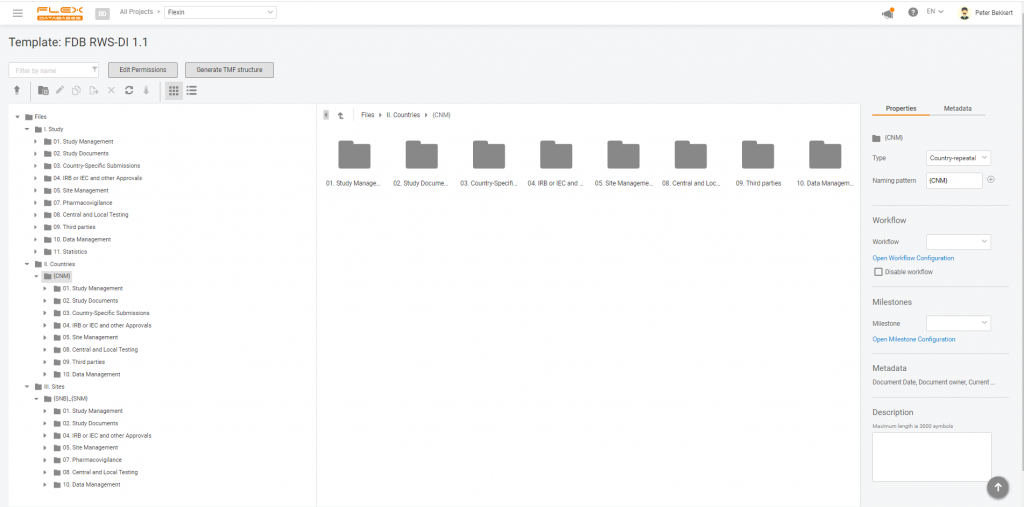
As we all know, the eTMF structure for RWE studies differs from a general TMF Reference Model.
Here’s the list of main differences:
To make everyone’s lives easier, we added a template for RWS to our eTMF – you can select it when you create an eTMF structure for the project from the list of default templates.
There are five main aspects to the template that we’ve included to make sure, that the process is as smooth as possible:
- Standardization: A template ensures standardization in the format, structure, and content of RWE documents within the eTMF. It provides a consistent framework for capturing and presenting real-world data, facilitating comparability and analysis across different studies or projects.
- Data Integrity: RWE documents often involve complex data sets and analyses. A template helps ensure that the data and analysis methods are documented consistently and accurately, maintaining data integrity and reliability.
- Efficient Data Collection: A well-designed template streamlines collecting and organizing real-world data. It provides predefined sections and prompts for capturing relevant information, making it easier for researchers and data analysts to structure and input the data efficiently.
- Collaboration: RWE often involves collaboration between stakeholders, including researchers, clinicians, data scientists, and regulatory professionals. A template facilitates effective collaboration by providing a common language and structure for sharing and reviewing RWE documents, ensuring clarity and understanding among team members.
- Quality Control: Templates serve as quality control tools for RWE documents. They enable consistency checks, peer review processes, and verification of adherence to predefined standards. This ensures that the RWE documents meet the desired quality criteria before being finalized and included in the eTMF.
Here’s how it looks like:
So now, when working in Flex Databases eTMF, you can set up your RWE study in minutes and eliminate the burden of structure creation from your team.
Reach out to our BD team at bd@flexdatabases.com t



