Site Health Assessment as a part of Risk-Based Monitoring
June 30, 2022
Risk-Based Monitoring (RBM) has become one of the trends in clinical trials of the last decade. Sponsors and regulatory authorities recognize RBM as an efficient approach to ensuring patient safety and data quality. We always keep track of market trends and develop features that facilitate our clients’ needs in implementing RBM techniques.
What is Site Health Assessment (SHA)?
Site Health Assessment is a process of differentiating sites to achieve a more targeted monitoring strategy. Top-performing sites can be monitored less thoroughly, whereas low-performers need more intensive monitoring and detailed attention.
In general, Site Health Assessment is based on a questionnaire completed by monitors after onsite visits. Monitors assess different quality aspects of the site, such as site personnel involvement, PI oversight, and document quality. Once all answers are collected, the system calculates the overall score and assigns the health level (high, medium, low) per the predefined thresholds. This health level is considered when adjusting the frequency and extent of monitoring activities.
How does SHA work in Flex Databases CTMS?
There are several steps to conduct SHA in our CTMS.
Step 1. Create the questionnaire to perform SHA.
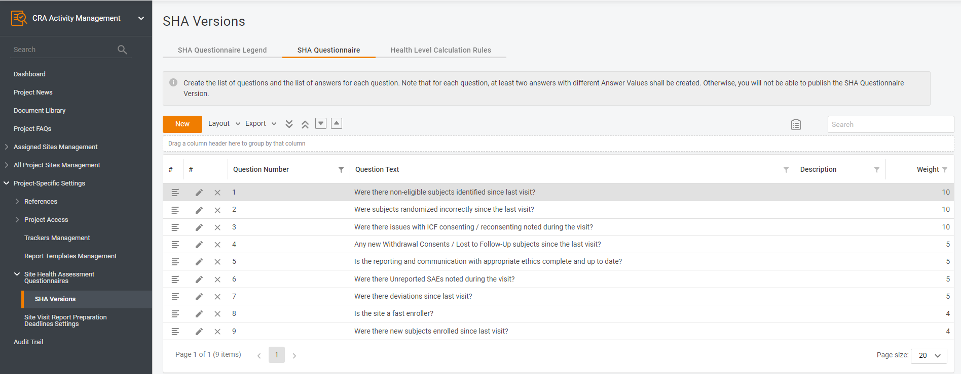
There is already a questionnaire template in the system which you can use, but the template is entirely configurable. Each questionnaire question has a different weight, as various aspects may have a different impact depending on the study phase and stage, and the weight may vary from 1 up to 30 points. The more points the site gets, the fewer risks it has and the greater quality and performance it shows.
Important note: If you need to change the template during the study, you can copy the previous version and amend it as needed. Say, when getting close to Database lock, you could get the weight up for the questions related to query resolution and ISF maintenance.
Step 2. Add the questionnaire to the monitoring visit report.
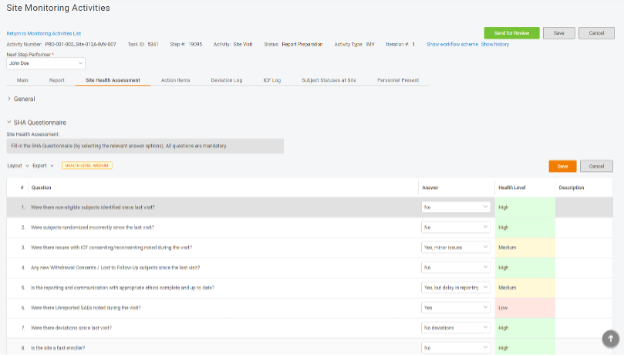
Make the questionnaire a part of site visit reporting so CRAs can give their qualitative assessment when completing reports. With a single click, CRAs can answer the questions, and the system will compute the score.
Step 3. Let the system take care of the result’s calculation
When all answers are submitted, the system will take its turn and calculate the SHA based on the provided data. If CRAs perceive the health level to be different from the automatically calculated score, they are free to provide their suggestion in addition. As a result, you will have a holistic view of the site health level and be able to calibrate the template in the future.
Step 4. Check the results of your SHA at both site and study levels
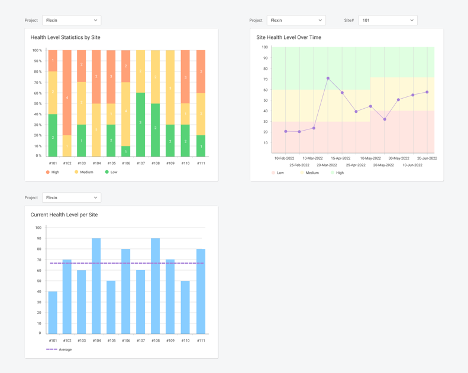
Let the Project Manager or Project Lead make informed decisions based on the results of SHA in our CTMS. On one screen, you would see, which sites are overperforming or underperforming, and which of them require an additional visit or training. It’s no longer a guess.
The whole process of SHA is straightforward. You don’t have to be a Ph.D. to set it up. It does not take more than a couple of minutes for your CRAs to fill out the questionnaire, and at the same time, it reduces the risks and time potentially spent in case of missing underperformance of the sites.
Keep your studies on track with our CTMS – send us a request to bd@flexdatabases.com to learn more.



