How do we make validation simple and speed up implementation up to 2 times
July 6, 2021
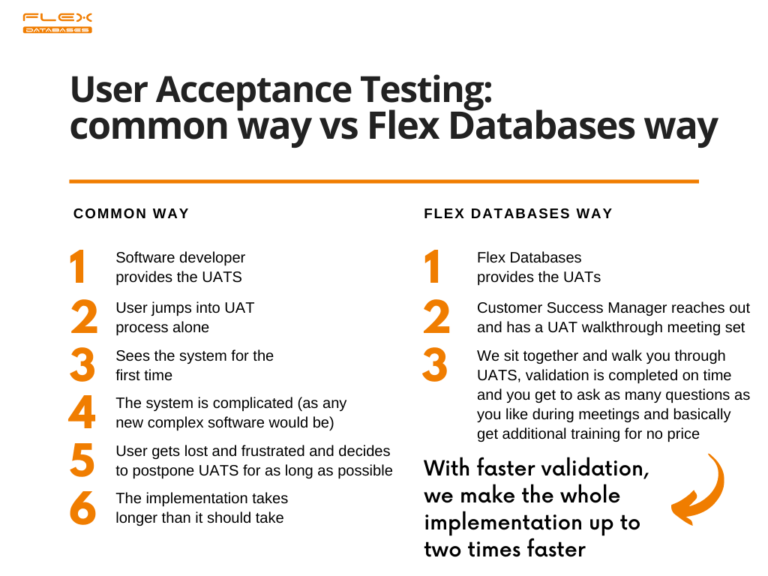
Software validation is tricky, especially for those who’s never met it before. Still, it Is required, and it can affect implementation timelines in the worst way if handled poorly.
That’s why we at Flex Databases developed a simple workflow to make validation almost effortless and as simple as possible:
Step 1: We provide the happy new customer with the validation package, including core functionality and installation qualification documentation.
Step 2: We walk you through the end-user validation step.
First, we provide User Acceptance Testing scenarios (UATS) to you. UATS are long documents with a detailed explanation of what the system should do for you and how it should behave. All you have to do is follow the testing script, tick every step on the way, confirm that the system behaves how it’s supposed to behave and make a validation report at the end of the process. Sounds simple enough?
But proven to be complicated to navigate in a new system.
Thus, a dedicated customer success manager will sit with you and walk you through User Acceptance Testing online! It only takes several online sessions, where you go through the scenario together.
Our approach allows us to speed up the complete implementation process almost up to two times and provide you with painless validation, which is rare in our field.
Step 3: Profit. You have a validated system and a complete documentation package for you to use at any time during audits or inspections.
Bonus information point:
We get a lot of questions like “What’s an end-user validation, and why do we need that, and finally – do we really need it?”. According to applicable regulatory requirements sponsor is responsible for ensuring that the system used is reliable and – yes! – has to validate it.
Validation does not have to be a burden. Validation with Flex Databases is not a burden.
Sounds like something you’d like to try? Send us a demo request through any form on this website, or drop us a mail at bd@flexdatabases.com



