Solving site payments problem with Flex Databases Subject Tracking & Invoicing
April 2, 2021
st trial sites work with no more than a 3-month operating budget. With an estimated two-thirds of trial sites falling into this category, software companies have sought to close this gap in effective financial operations with process automation and payment schedule. So CROs, pharma companies, and biotech can finally get transparency in site payments.
Likewise, effective subject management is essential for clinical trials. Sometimes this means coordinating across several physical locations, and other times it means the organization of large groups of people. No matter what the problem is, eSystems are there to solve them all.
What are the exact problems, though?
- Overall process complexity
- Human factor with budgets and invoices
- Manual work requires a lot of time
- Changes when a study is in progress
- Lack of transparency
- Disorganized payments systems
- Delayed payments – frustrated sites
Flex Databases Subject Tracking & Invoicing module solves ALL of them.
Simple process for complex issues
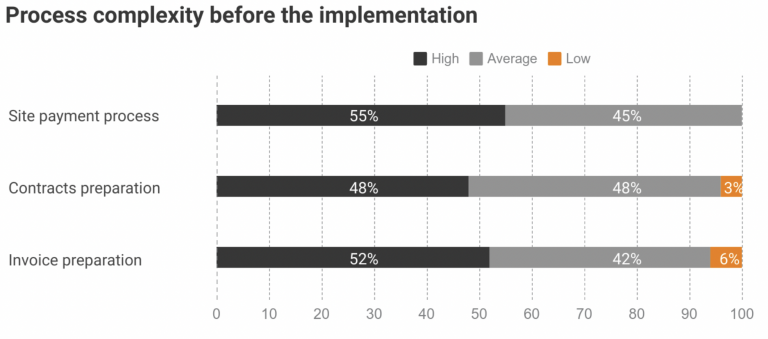
One of our customers, Novo Nordisk, implemented Subject Tracking & Invoicing module to find a solution for growing payment processes complexity and associated burden. Before the implementation, the company has dealt with difficulties and delays in payment processes:
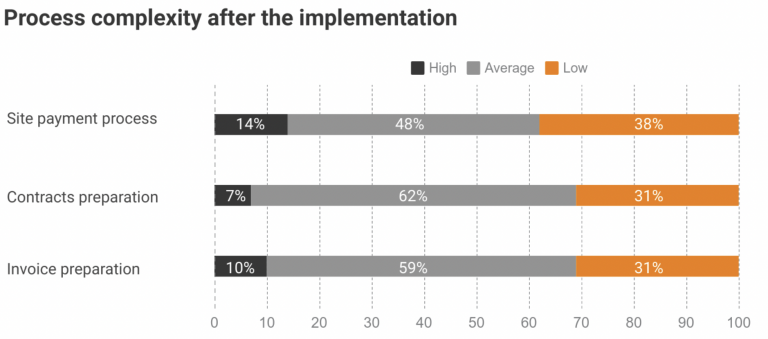
After implementing the Flex Databases solution, Novo Nordisk employees were able to speed up and simplify the site payment process, such as contract and invoice preparation, and lower the workload of responsible roles. Here is the result of the same questionnaire about the overall process complexity a year after Flex Databases CTMS implementation:

Financial Accountability & Transparency
Reports and predictability of payments and budgets.
With the ability to track and export all investigator records on payments, you’ll have all the data needed to report on payments into sites and see the real picture. No more multiple systems and spreadsheets to report and reconcile!
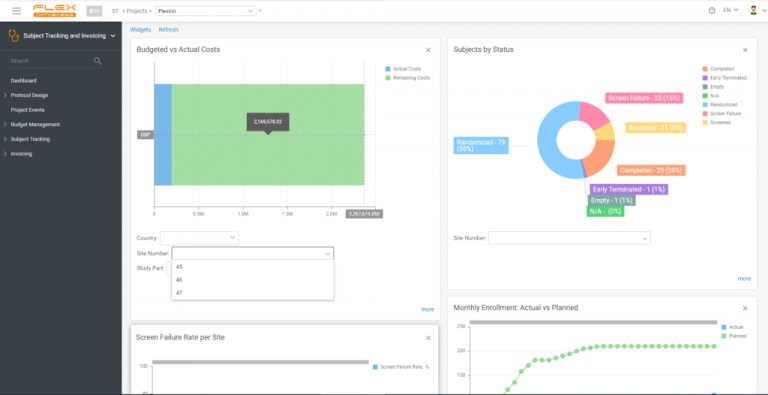
Payment Process Speed-Up
With automated invoice generation based on EDC data, you can create invoices right after site work is complete.
Sites are Happy and Engaged
Enhance your site relationships with quick, on-time payments with no delays or errors. Hassle-free payments to sites are an advantage for both sponsors and CROs.
Endless Scalability
You control sites’ budgets and all the budget rules on any level. It’s no longer just study or country-specific. Use site and budget version-specific invoicing.
Does our Subject Tracking & Invoicing like something you’d like to try? Send us an e-mail to bd@flexdatabases.com or request a demo via the button on top of the page to schedule!


