A simple way to speed up site payments & grow site engagement
April 26, 2023
How long does paying to the sites while running a clinical trial take you? Average figures usually range from 4 to 6 months, even though in the contract, the payment period would typically be a month.
Will you get frustrated if you are paid with a half-year delay? Yeah! Will that motivate you to work more with such a partner? Doubt that.
On-time payments motivate the sites to recruit faster and meet their enrolment targets. And we know how you can get on top of your site payment game: switch to per-procedure payments.
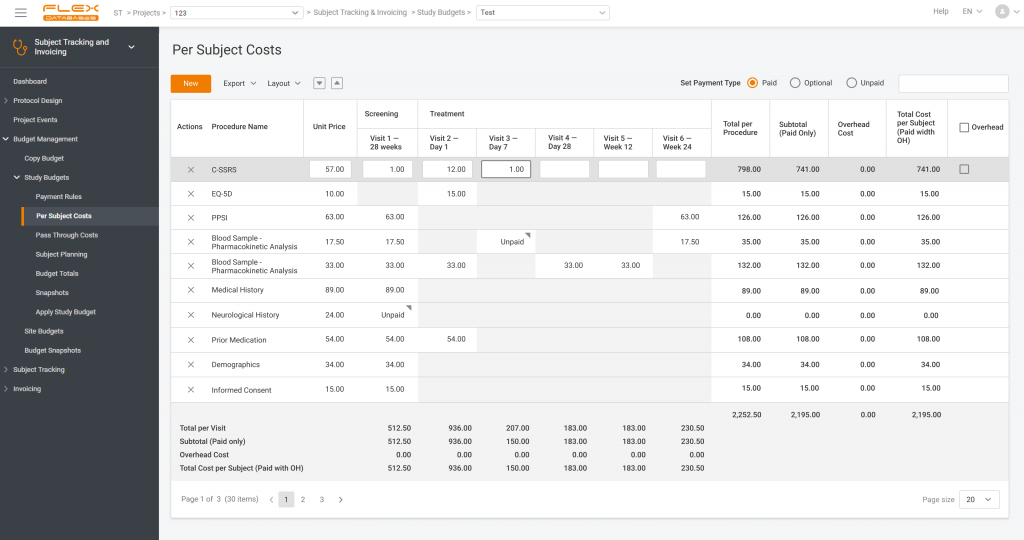
In CTMS, site payments are based on visits, subjects, or procedures performed. We pioneered implementing per-procedure approach in our Subject Tracking & Invoicing module, a part of CTMS. It’s nothing new now, but we believe that we still have the best one.
Here’s an example of how it works:
- The site performs blood sampling or measures the patient’s temperature.
- The system catches it.
- The site gets payments based on this information.
As a result of implementing per-procedure payments, our clients improved the timelines of site payments dramatically and met the contracted estimates of 1 month. That pushed the sites to enroll patients into these studies faster, preferring these Sponsors over the others. Novo Nordisk is an excellent example: they were able to speed up their site payment processes eight times & drastically improved relationships with sites and investigators.
Do you want to be among the sites’ favorite clients and enroll subjects rapidly? Reach out to our BD team and learn how to do that.



