Streamline learning management of any scale with Flex Databases LMS
June 1, 2023
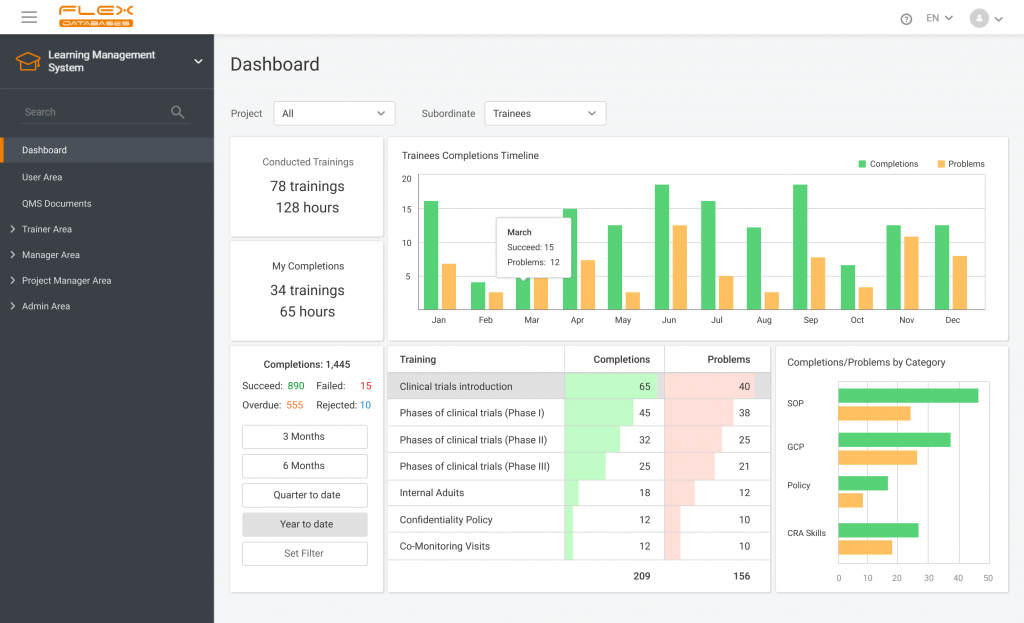
When working in a regulated industry, learning management is a huge concern since the need to follow SOPs & complete training applies to all company employees (at different levels, but still). It’s complicated enough when we think of dozens of people split between dozens of roles, but what if it comes to hundreds or thousands? Streamlining the learning management process of thousands sounds like a nightmare but worry not – we’ve got you covered.
These are the five simple features of Flex Databases LMS that’ll help you to get on top of your learning management:
- All major training material formats are available within one system. Do you want classics, such as docs, pdfs, or presentations? We’ve got you covered. Want to share fancy videos or up the game with quizzes? Be our guest. No need to maintain & manage several different systems – you can have it all with one.
- Batch assignments based on selected criteria to make the continuous process as simple as breathing. You can assign new training based on basically any criteria your employee profiles have – location, role, seniority-based, etc. One office or twenty – if you need to spread a new global SOP, it only takes a few clicks.
- Training matrix to simplify onboarding. Again, you build different matrixes based on roles, departments, locations, etc., so whenever new employees come on board, they automatically receive everything they need. You can have one matrix, ten, or a hundred – a system can support the needs of an organization of any size.
- Quizzes are here for you to make sure that employees are actually reading through the training material. The system won’t let users mark training as “complete” until the quiz score (also completely configurable) is high enough. You can set up question randomization as an additional protection level to ensure nobody shares answers.
- The system will take care of notifications to ensure that nothing falls through the cracks. You take care of one thing – selecting the frequency, and the system will do the rest.
Ten, a hundred or thousands of employees – all covered by one system. Do you want to learn more? Visit the LMS product page, request a demo through the form at the top of the page, or email us at bd@flexdatabases.com.


