The scope of the #SCOPE2019
February 27, 2019
10th Annual SCOPE Summit was held on 18 – 21 February in Orlando, Florida.
If you have missed it for any reason, we are here to fill you in!
Usually, all the insights we gather through an event are of two kinds: current industry problems and upcoming trends. This time we begin with Problems.
Main problems raised:
- Study speed (study start-up, site selection, patients enrollment, etc.)
- Study cost
- Data Bulk
- Visibility/transparency and predictiveness/forecast (metrics, KPIs, dashboards)
- Multiple systems used for one study lead to lack of transparency
- Delayed actions/responses
- Unified vs. Integrated (the systems should be integrable)
- Design study the sponsors/CROs wants to do, not the way technology imposes
Main problems of payment process raised:
| General: | For sites: | For sponsor: |
| Data quality Payment frequency Negotiating Invoice generation speed | Cash flow Financial visibility Administrative burden (revisions, reparation, etc.) | Payment Accuracy (overpayment) Global financial visibility & control Predictability Processing and adjunction Support burden |
And continue with Trends:
Main trends discussed:
- Virtual (Remote/Decentralized) Clinical Trials
- Focus on outcomes, not inputs
- Predictive analytics in Clinical Trials
- Metrics (ICH E6 R2 requires use of metrics by sites and sponsors)
- More use of outsourcing models
- Partnership/Collaboration – treat providers as partners
- Without executive support and sponsorship changes aren’t possible
Several presentations were dedicated to Remote/Virtual Clinical Trials theme. The main benefits of virtual trials are:
- Decrease in the number of investigator sites
- Decrease in price of procedures and site staff
- Decrease of start-up fees
- Increase of software and device use
Remote Clinical Trials is trending, as it allows to cut the costs of trials.
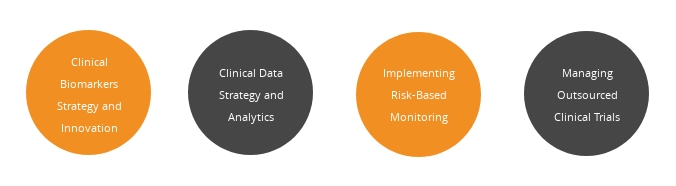
Lead sessions by pharma/biotech speakers:
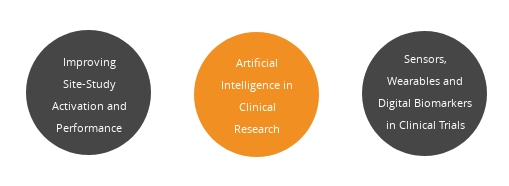
Lead sessions by vendors:
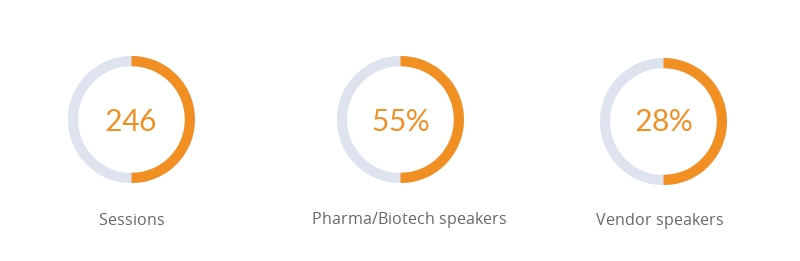
General statistics:

See you there next year!



