What’s next for Flex Databases Learning Management System?
March 11, 2021
It’s been two years since our How to stop worrying and start working in clinical trials material dropped.
Content-wise, it was the highest point for our Learning Management System so far. I have to admit, since then, we kind of abandoned its storytelling activities and just sometimes briefly mentioned new developments for LMS in our release notes. Shame on us, Flex Databases Marketing team.
But the good news is our Development team never did such a thing!
So, here’s a list of what’s coming for Flex Databases LMS:
Major design update
- First of all, the design will be changed throughout the module to make it even more convenient for users and easy-to-navigate
- The module reports will be redesigned to become more informative with more visual elements
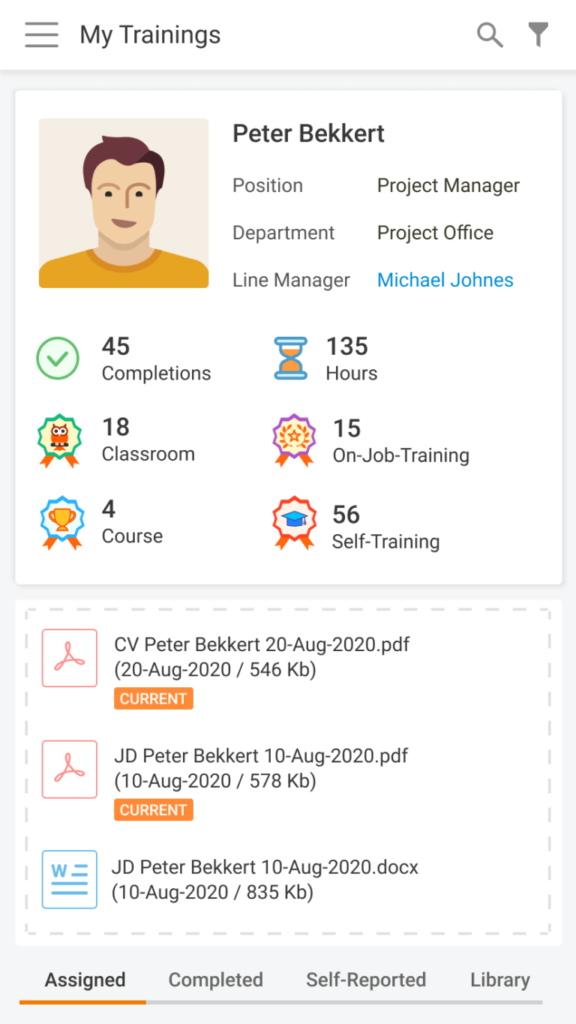
- The new User Area will aggregate several sections used in the previous version of the module
- So, the current training assignments, the available optional training, the self-reported training, and the training history can be viewed in the same place
- The module reports will be redesigned to become more informative with more visual elements
As you can see in this mock-up, this section provides a clear overview of the pending training’s progress with the possibility to set various filters that allow searching quickly:
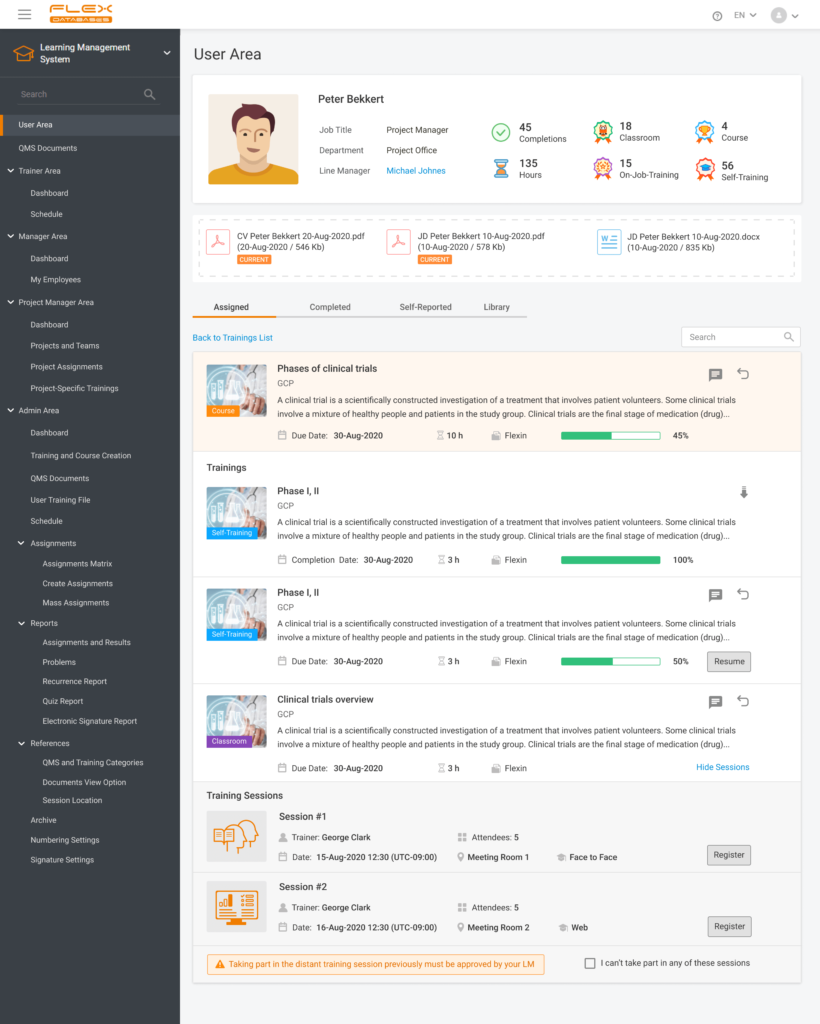
- The System will support even more various formats for the training materials applied to the training. For example, it will be possible to use the training materials designed in SCORM.
This is the training example that we see with the eyes of a trainee. We can open up the training material and use the SCORM material in a separate window. Here we have a quiz with the results assessment summary – number of attempts, grading method, and the current grading:
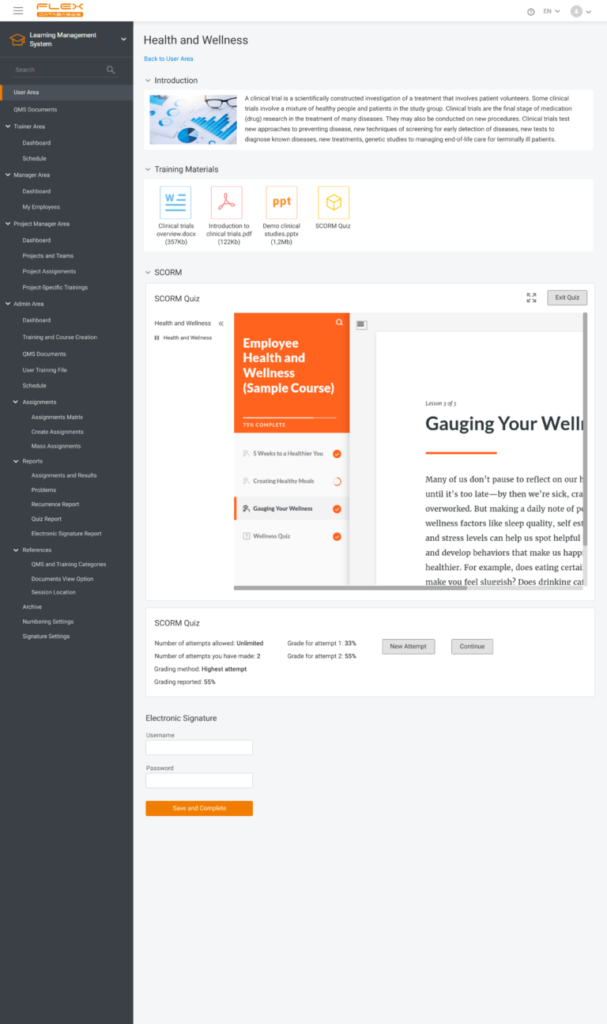
- Training quizzes will become more diverse and flexible. This makes it possible to shuffle the list of questions for each user, to set the maximum number of attempts to pass the quiz, or, on the contrary, to ease the training completion by highlighting the wrong answers.
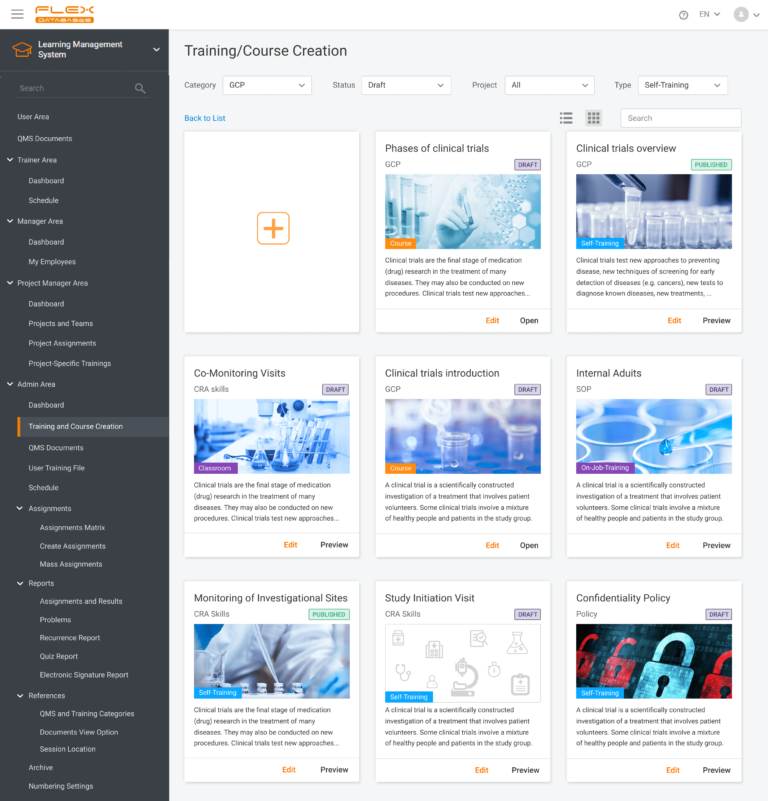
- The training creation process will also change. Self-trainings, trainer-led training, and training courses will now be created at the same location.
From the Administrator’s perspective, we can open and edit drafted or published training in this section:
- Extended reporting possibilities – more infographics, more widgets based on the module reports will be available for module Admin, Line, and Project Managers. It is easy to see what training is the most problematic for employees – by the quiz failed attempts, overdue, and rejections of the scheduled training sessions.
- Last but not least – the long-awaited mobile adaptive version for all users to always have LMS handy:

Does Flex Databases LMS sound like something you’d like to try? Send us an e-mail to bd@flexdatabases.com to schedule a demo or simply fill a form via the button on top of the page



