Synchronizing safety data from EDC/eCRF with Flex Databases Pharmacovigilance
April 21, 2022
Many years since the appearance of different platforms and systems for clinical trial operations, there has been a question about making all the systems work synchronized. For most parts of the platforms and systems, it occasionally became possible, though Drug Safety Specialists still have to lead their pharmacovigilance processes independently.
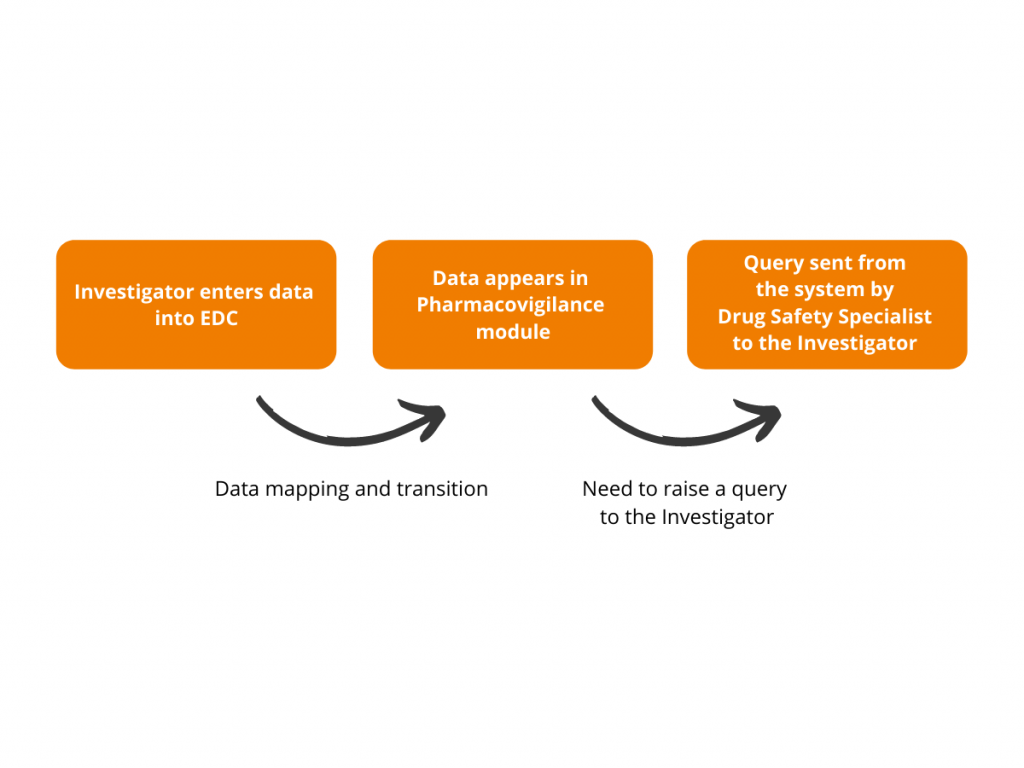
Having the objective to streamline safety data processing and simplify the lives of Drug Safety Specialists, Flex Databases is ready to arrange the automated data flow from any eCRF/EDC used by Sponsor or CRO to our Pharmacovigilance module. So that any data capture or update made by Investigator in eCRF is automatically transferred to Pharmacovigilance module for further analysis and reporting. Moreover, not to burden Drug Safety Specialists and avoid manual reconciliation, we created customization enabling the system to grab only the reactions applicable to expedited regulatory reporting: SARs and/or SUSARs. In this case, Drug Safety Specialists get into the Pharmacovigilance module only for the cases they genuinely need to process. Though in case our clients might need all AE transition, we can easily assist with such kind of full information flow as well.
This is how it works:
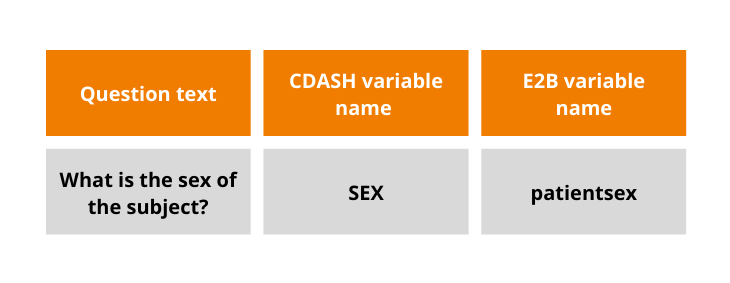
One of the major challenges while mapping the systems is the difference in standards used for EDC and Pharmacovigilance – CDASH and E2B, respectively. Having this in mind, Flex Databases developed a mechanism for mapping data elements originating from these two standards, thus avoiding human mistakes. While transferring the information from eCRF to Pharmacovigilance module, the system automatically matches the data elements and transfers information through the mapping tool developed and programmed by our team.
Data mapping:
As soon as Pharmacovigilance module receives new or updated information from the eCRF system, it triggers an email notification to the Drug Safety team. It ensures Drug Safety Specialists have a heads-up and can promptly initiate a regulatory reporting process, avoiding any difficulties and delays in communication with Regulatory Agency.
Last but not least, the SAE reconciliation process has always been a big issue for companies with clinical and safety databases not talking to each other. With automated data transfer provided by our platform, we can reduce, if not eliminate, the risk of discrepancies between the two databases since the eCRF now serves as a single source of truth for safety data. Any updates made in eCRF are carefully audit trailed in the Pharmacovigilance module. If the Data Management team is required to run an SAE reconciliation report, then our tools are at their disposal. They can easily export each and every data element from our Pharmacovigilance module in either human- or machine-readable formats.
To simplify the communication process between Drug Safety Specialists and Investigators, we developed an option of sending queries to sites right from the Pharmacovigilance module. The Drug Safety team can send the clarification requests and questions to the investigators, keeping the whole discussion trail tied to a certain data element.
Raising a query:

We are more than sure that these new features are the future for the whole industry as it makes pharmacovigilance easier and avoids double data entry into the systems. Flex Databases is proud to be among the first companies of eClinical trial solutions to offer the synchronization of eCRF and Pharmacovigilance modules for our partners.
Reach out to our BD team at bd@flexdatabases.com to learn more about Pharmacovigilance module or to schedule a demo.



