How CRA Activity Management impacts daily life of a CRA
March 17, 2020
If we imagine clinical trials as a human body, Clinical Research Associates would be the blood cells. They travel a lot across the whole organism, delivering data oxygen to all organs and body parts. If something stops the proper functioning, it may result in global failure. That’s the reason why we developed CRA Activity Management module – to help our clients ensure and support the proper CRA’s functioning.
From hours to minutes
Optimization starts with routine tasks. It usually seems like routine is effortless and not time-consuming, but it might take away the most significant part of the day.
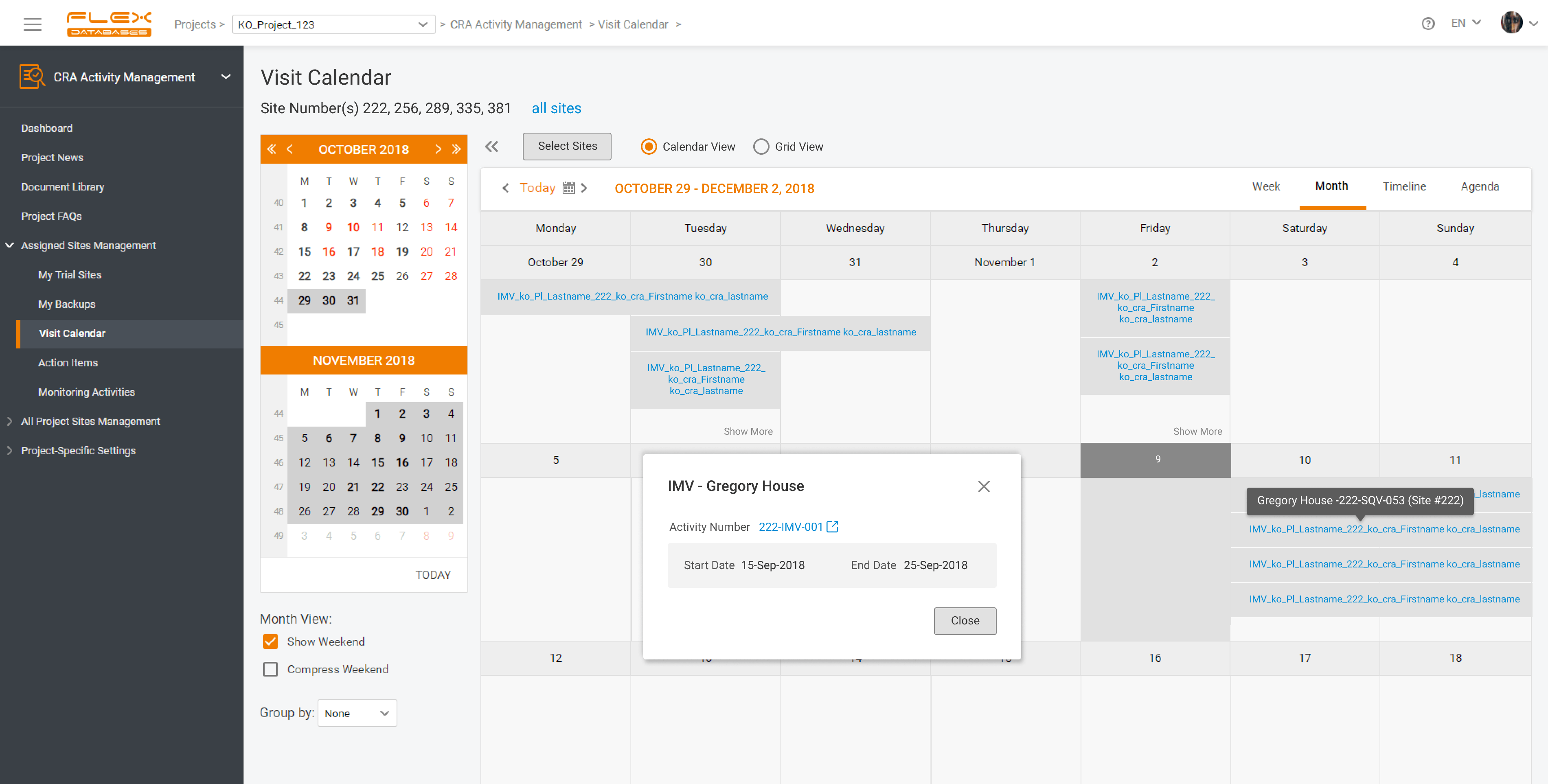
With Flex Databases CRA Activity Management you’ll get:
- Customizable widgets on the first screen: simple tracking of the most critical metrics of user’s choice.
- All Monitoring Activities stored in one place: instead of checking multiple task managers, CRA can follow one, with tasks lined up by deadline (upcoming visits are included).
- Simple site visit planning and scheduling: centralized calendar to oversee workload and plan visits accordingly.
- Customizable templates for confirmation and follow-up letters: push a button in the system and get your letters done in 1 second, the system will take care of the rest.
- Simple contact report creation.
- All documents are filed into TMF automatically and immediately after completion.
By organizing the routine, Flex Databases CRA Activity Management helps to reduce the amount of time spent on it from hours to minutes or even seconds, if we talk about fully automated features such as TMF filing.
Reporting made easy
CRAs manage multiple sites at once, and with such workload, it’s easy to get lost and fill in a wrong report. The system helps to maintain and decrease the number of errors caused by the human factor by organizing information clearly and simply.
- The system picks the type of visit report according to the type of the visit: PSV, SIV, IMV, COV.
- Automated notifications remind about reports that must be written, completed or passed the review to keep the user aware of the actions that need to be done in the future.
- Offline reporting possibility is aimed to help road warriors keep on going even when the connection is poor or non-existent (on a plane, for example).
- All required fields are marked, and it’s impossible to submit an incomplete report.
- Inbuilt problem control: action items, protocol deviations tool keeps track of all the issues, occurred with sites.
- Ad-hoc reporting tool for cross-project and cross-module reporting (graphs, widgets) – all the data the user might need is on the table in a simple click.
CRA’s life is not the simplest one, but it surely can be more comfortable with CRA Activity Management.
To know more, request a free demo via button on top of the page or send us an e-mail to bd@flexdatabases.com.



