How we grow our product but not prices through the years
October 22, 2021
When you decide on the eClinical system or any other software provider, there are many questions to ask – compliance, efficiency, security, support, etc. And at the end of the day, it is also how much I pay vs. what I get for it?
What about the money?
How did SaaS pricing grow in proposals for the past three years? For the total of 0%.
How we grow our product
We’ve brought the Flex Databases platform to a whole new level during the last three years, and the development process never stops.
Just a few examples of significant changes over the last three years:
System-wide:
- Complete system design update to ensure the highest level of usability
- Report Tool to make 100% of your data work
- Customizable workflows for you to set up existing processes however you’d like
- API
CTMS:
- Configurable trackers, templates, workflows, customizable fields – everything under your control
- Offline reporting
- Multi-currencies for different budgets and beneficiaries inside of the same project
eTMF:
- OCR
- Emailing documents in and out of the system
- Metadata auto sourcing
Pharmacovigilance:
- Neural network for signal detection, medical coding
- Direct submissions to the world’s largest regulatory authorities
- XML import/export
… and it’s just a tiny part. How tiny? Let’s see!
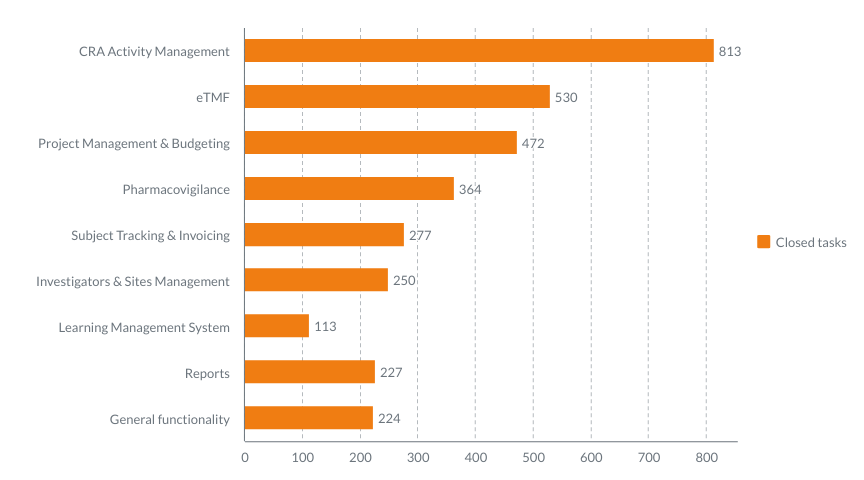
Here’s the number of tasks closed by our Development teams during the past three years. Under the “task,” we mean a feature or an improvement to the module. So, for example, CRA Activity Management module got a total of 813 features and improvements during the past three years. Sounds impressive!
Perspective is important. Comparing the huge resources that go into our development and the price described above, where do we get the funds? The key is the growing number of clients!
Want to become one of our clients? Request a demo through a button on top of the page or simply send a request to contact@flexdatabases.com.



