Keeping Critical Data Safe and Accessible Using eTMF Technology
January 11, 2018
In an age where nearly a hundred percent of your clinical research organization’s data can be stored using paperless resources and the number of cloud based solutions on the market has become significantly larger, the electronic trial master file has emerged as one of the most relevant new developments in the world of clinical research and pharmaceutical technologies.
What is an eTMF?
The electronic trial master file, or eTMF, is a formalized method for storing the entire range of images, documents, and other digital content associated with your organization’s clinical trials in a way that is compliant with the applicable government standards, laws, and conventions for records management. What that means is that you keep your paperless data as safe as the real thing and invulnerable to malicious attacks on company infrastructure which could compromise operationally crucial information and at the same time increase your CRO’s productivity because of the amount of man hours that you can save on document organization and audit preparation.
The purpose of the trial master file is to give any organization a way to facilitate collaboration between team members because it acts as an all-encompassing directory of clinical trial activities and provides easy access to the appropriate materials during an audit. The beauty of the eTMF is it offers the same advantages, but is also accessible from anywhere on the planet using nearly any internet-connected device, and includes the protection of digital information.
Integrating an eTMF System
Every operating Clinical Research Organization (CRO) is required to keep a trial master file, but a growing percentage of those operations have already transitioned to an eTMF system in order to make their operations more effective and save money on printing and paper costs.
The primary motivation to begin using an eTMF system is the astounding increase in efficiency that you will see after you start using it. The electronic trial master file automates a number of business processes by storing the entirety of your CRO’s information in a central database that is accessible to staff throughout the organization. In order to access the system, an employee needs any internet connected device and the proper login credentials. Modern eTMFs offer role-based access that you control and track system activity so your documents remain safe. The eTMF reduces the liability that your organization is exposed to by making sure that you are in line with the applicable regulatory compliance requirements and document management best practices. Additionally, the company’s overall organization and efficiency is increased because automated systems are demonstrably better at keeping critical operational documents in order and ready to use at a moment’s notice. Simply put, automated processes reduce the number of errors that occur by reducing the amount of data entry tasks that need to be completed.
Electronic trial master file systems improve your team’s productivity by acting as a secure homebase for everyone to coordinate and communicate. Documents are shareable and viewable anytime, anywhere, and from any device. The reach of the eTMF is truly close to becoming unlimited in its ability to keep teams connected from any place on the globe. Collaborative efforts that rely on communication back and forth between team members are notorious for seeing a breakdown in the process that results in a slowdown of the entire operation. With an eTMF, that process is streamlined because the system connects relevant employees and supplies notifications when they have tasks which require their attention.
The audit process can be dramatically shortened when completed automatically. Anytime a user modifies a document in the eTMF, that modification is logged by who did it, what they did, and when they did it. This automation means that your employees no longer need to track their own modifications, the system does it for them. During an audit process in the clinical trials industry, one of the most significant metrics tracked is the chain of document custody. With an eTMF your company is always ready for an audit and external inspections will yield more predictable results because the only preparation that your company will need is reviewing what you have in the system already. Take the headache out of the audit process.
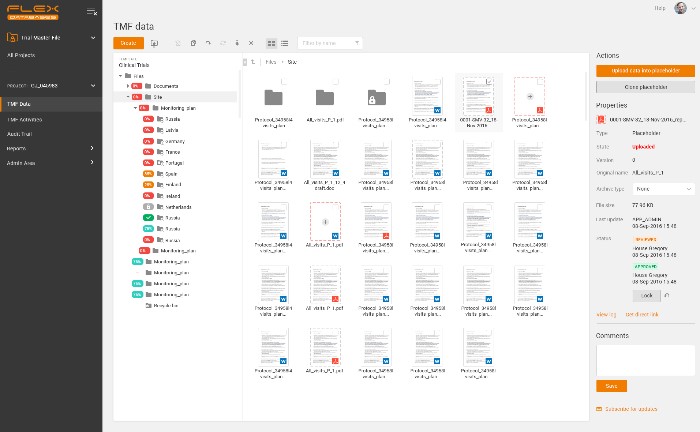
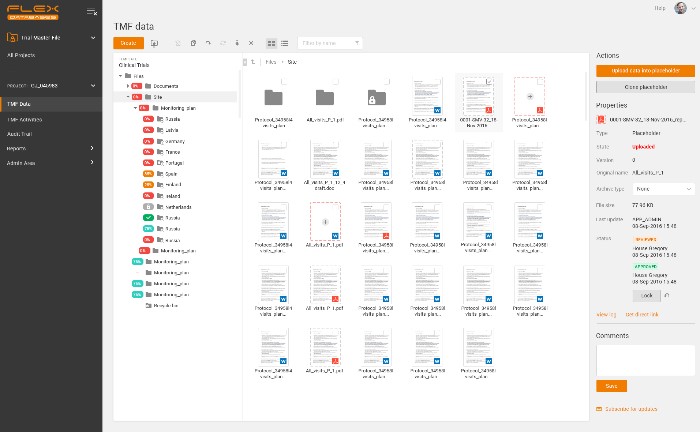
Example of eTMF structure – all in one place
Technology of the Future
One of the fastest progressing sectors of the tech world right now is inarguably machine learning and artificial intelligence. These mechanisms are simply the byproducts of complicated algorithms that mandate how a computer receives or sends information, but they are already having a very real impact on the world of clinical trials. Everything from computers observing the reactions of chemical processes, to sophisticated disease recognition technologies that detect abnormalities far more accurately than their operators. The influence technology is constantly being expanded as it makes room for more improvement in clinical trials management, and the eTMF is a perfect example of why. The eTMF has a number of functions which not only increase productivity but accuracy as well. A robust eTMF should contain the following functionality:
Document Upload and Creation: create a new document within the eTMF interface or upload an existing one some that teams can collaborate on projects in a secure environment like never before.
Classification, Indexing, and Storage: documents should be classified and stored in a way that is completely customizable so that the eTMF remains uncongested and clearly organized.
Robust Security: your eTMF should be beyond reproach from malicious actors and attacks. Ensure that your essential documentation is not only safe from external threats, but internal threats as well with privileges and credentialed access throughout the entire system.
Quality Control: make sure that your documents are ALCOA (Accurate, Legible, Contemporary, Original and Attributable) compliant throughout the entire module with automatic proofreading and document analysis features.
The Audit Trail: always be aware of which employees are doing what inside the eTMF system by tracking modifications, uploads, and deletions automatically. Save countless man hours organizing an audit, because it’s been completed as it happened.
Reporting: automatically generate reports using information stored in the eTMF, additionally, reports can be generated about the information stored in the eTMF itself such as tracking metrics like document upload rate by user and other.
If you are planning to integrate an eTMF solution – contact us and we will guide you through all the aspects of it.



