United States-based Tranquil Clinical Research recently signed Flex Databases as a software provider for Clinical Trial Management System and Pharmacovigilance management solution.
Tranquil’s ultimate goal is excellence in the clinical trial process and bringing trustworthy products to patients.
Flex Databases CTMS and Pharmacovigilance system are well-known as unified yet flexible eClinical platform for full-cycle clinical trial management.
- It helps to optimize and automate the most important aspects of any clinical trial process – monitoring, grants & subjects, and sites
- It provides easy capability for financial management, study monitoring, feasibility assessments, and other needs
- It is a secure and validated PV system that serves as a single point of entry, assessment, and reporting of safety data
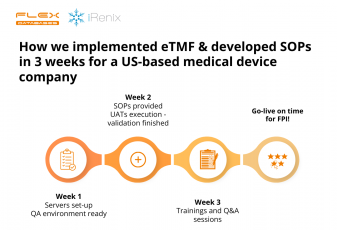
Baseline implementation takes only 5 weeks!
Tranquil clinical research was created to ensure that the entire clinical trial, device, and drug development process is conducted with ethics and the patient in mind first and always. The business model ensures a client’s drug, device or investigational product in the clinical trial is adherent to the highest level of quality and integrity in the services company provides.