Electronic Data Capture – Everything You Need To Know
July 22, 2025
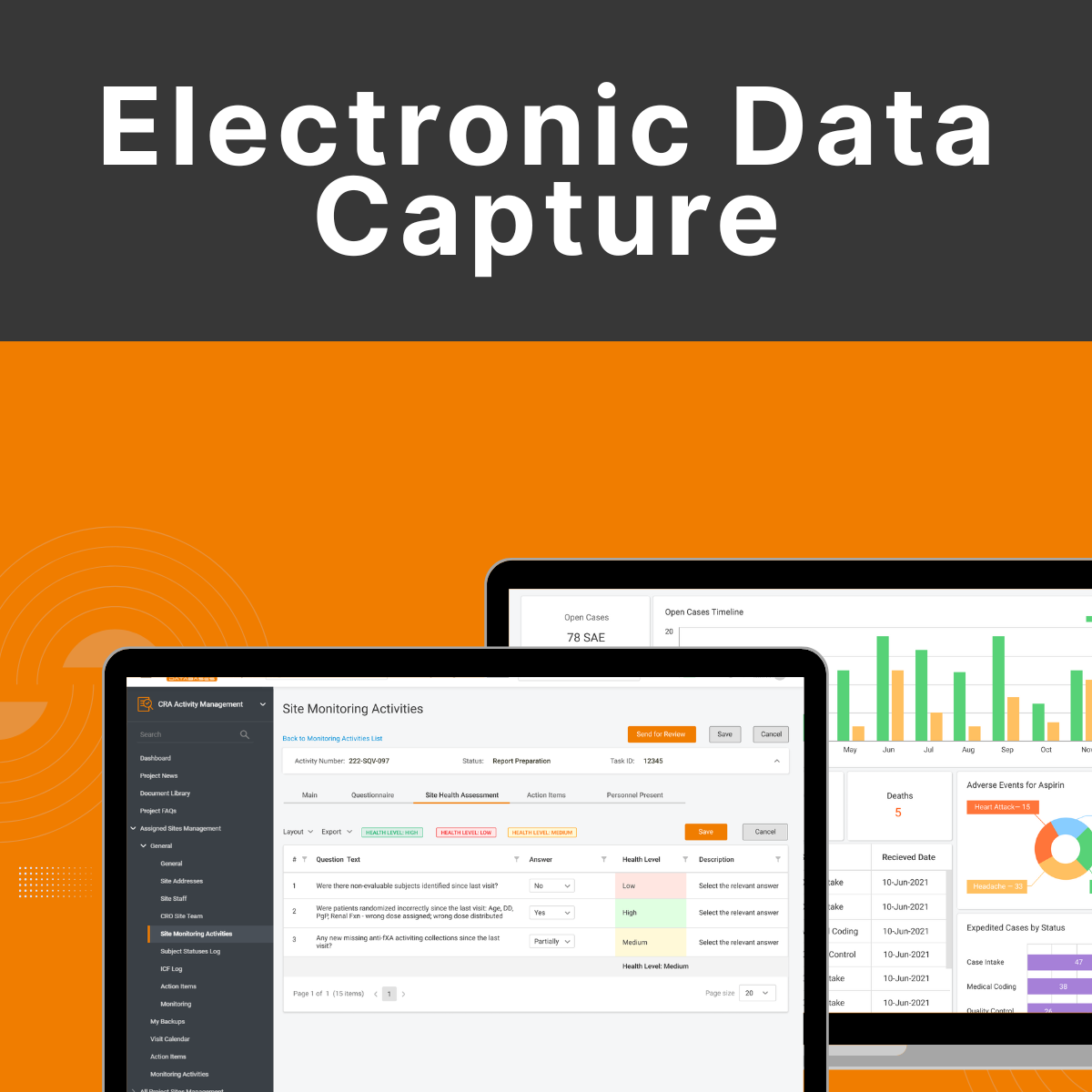
What is Electronic Data Capture?
Electronic Data Capture (EDC) is an electronic system applied to clinical trials for capturing, managing, and storing patient data. Instead of paper-based forms, investigators enter clinical trial information directly into a secure, web-based site. EDC systems validate data integrity, reduce errors, and automate the data collection process – accelerating clinical trials, making them more efficient and regulatory compliant.
What is The Role of EDC?
Electronic Data Capture (EDC) in clinical trials primarily automates and secures the collection, validation, and management of clinical data. Clinical sites use EDC systems to enter patient data directly into electronic case report forms that are stored in a central, web-based database. This allows:
- Real-time access to data for sponsors and monitors
- Fewer errors in data due to computer-automated validation rules
- Enhanced query resolution and better data quality
- Regulatory compliance like 21 CFR Part 11
- Effective data export for analysis and reporting
On the whole, EDC systems enhance the speed of trials, data integrity, and decision-making.
How Does EDC Work?
The system allows clinical site staff to key patient data directly into secure electronic forms after every visit. The forms are constructed from the study protocol and include in-built rules of validation to catch errors like missing values or out-of-range values.
When data is entered, they are available on the fly to study monitors, sponsors, and data managers, who can track progress in real time. If there are discrepancies, the system alerts queries, which site staff can deal with on the spot within the system.
Once data collection is complete and all queries have been addressed, the cleaned dataset is exported for statistical analysis and regulatory submission.
What Are The Different Types of EDC?
There are no specific types of EDC, but systems can differ based on structure, functionality, and deployment. Common distinctions are:
Cloud-based vs. On-premises
Most modern systems are cloud-based with easy access and scalability. On-premises are less prevalent but might be used for specific security or compliance needs.
Standalone vs. Integrated
There are some platforms that are exclusively data capture-driven, and others are CTMS, eTMF, ePRO, or randomization system-integrated end-to-end trial management solutions.
General-purpose vs. Study-specific
General-purpose tools have a wide range of studies with customisable templates. Some are specially or highly customized for certain therapeutic areas or trial structures.
Commercial vs. Open-source
Commercial EDCs are normally supplied with support and validation services, while open-source systems offer more flexibility in the form of higher in-house effort.
What is electronic data capture used for?
EDC is a key instrument in clinical trials, and its role varies slightly based on the role:
It is used by sponsors to monitor trial activity in real time, ensure data quality, and prepare for regulatory submissions. It gives them control over all study sites and more rapid access to clean data for analysis.
CROs (Contract Research Organizations) rely on it to manage data collection from numerous studies and sites. It helps them track data in a streamlined way, handle data queries in an efficient manner, and remain compliant with study protocols and regulation.
Sites (clinical research centers) use it to collect patient data accurately and efficiently after each visit. Edit checks are minimized by inherent errors, and query resolution and monitor communication are eased through the system.
In short, it guarantees timely, compliant, and correct handling of data at every phase of a trial.
What kind of data can you collect in an EDC system for clinical trials?
You can collect nearly all protocol-required clinical data, including:
- Demographics – age, sex, race, weight, height
- Medical history and concomitant medications
- Visit dates and procedures performed
- Lab results and vital signs
- Adverse events (AEs) and serious adverse events (SAEs)
- Dosing and treatment information
- Patient-reported outcomes (if integrated with ePRO)
- Assessment scores and test results
All data is structured to match the study protocol and is usually organized by visit and form.
How is data collected in clinical EDC software?
Site personnel enter data directly into electronic Case Report Forms (eCRFs) via a secure, web-based interface. Each form corresponds to a visit or study event and includes built-in checks to ensure completeness and accuracy.
Data entry usually happens after patient visits, but in some systems, data can be captured in near real-time especially when integrated with devices or ePRO tools. Once entered, the data is immediately available to sponsors, monitors, and data managers for review and analysis.
What are the main components of an EDC system?
A typical EDC system includes several key components that work together to support clinical data capture and management:
- Electronic Case Report Forms (eCRFs)
Custom-designed digital forms for entering patient data according to the study protocol. - Data validation and edit checks
Built-in rules to catch incomplete, inconsistent, or out-of-range entries at the time of data entry. - Query management tools
Allow monitors and data managers to raise, track, and resolve questions about specific data points. - User roles and permissions
Define who can view, enter, or edit data ensuring access is restricted based on study responsibilities. - Audit trails
Automatically record all changes made to data, including who made them and when, to ensure compliance and traceability. - Data export and reporting tools
Allow teams to export clean datasets for statistical analysis and generate reports for oversight and submission. - Integration capabilities
Many systems can connect with CTMS, ePRO, eTMF, or lab systems to enable seamless data flow across platforms.
What are the benefits of electronic data capture software?
Using EDC software in clinical trials has several unique benefits:
- Improved data quality: Automatic edit checks and validation rules reduce entry errors
- and missing data.
- Shorter data entry and availability: Site staff can enter data in real time, with immediate visibility to sponsors/CROs.
- Efficient query management: In-built functionality allows for easy raising, tracking, and quick resolution of queries.
- Regulatory compliance: Most systems enable GCP and 21 CFR Part 11 compliance through audit trails, user controls, and electronic signatures.
- Enhanced data security: Cloud-based systems offer encryption, role-based access, and secure backups.
- Improved data analysis: Clean, structured data can be exported quickly for statistical analysis and regulatory submission.
- Reduced paper burden: Eliminates the need for manual transcription from paper CRFs, saving time and reducing risk.
For what types of clinical trials edc can be used?
Electronic Data Capture (EDC) solutions are available for nearly every type of clinical trial, across a range of phases and therapeutic groups. Following is a classification of the main types:
By Trial Phase
- Phase I: First safety and dosage trials – EDC enables pharmacokinetic and pharmacodynamic data collection in an economical way.
- Phase II: Efficacy and side effects – EDC enables faster interim analysis and decision-making.
- Phase III: Large-scale efficacy trials – EDC provides scalability, data accuracy, and facilitates regulatory submission.
- Phase IV (Post-marketing): Data gathered from real-world environments – EDC enables long-term monitoring of data and reporting of adverse events.
By Study Design
- Randomized Controlled Trials (RCTs)
- Open-label Studies
- Single-arm Studies
- Crossover Trials
- Adaptive Trials
- EDC supports different designs by supporting flexible workflows, mid-study amendment, and real-time randomization.
By Therapeutic Area
- Oncology
- Cardiology
- Neurology
- Rare diseases
- Infectious diseases
EDC systems are adaptable with custom logic and forms to fit any indication.
By Study Scope
- Monocentric and multicentric trials
- Multilingual global trials
- Remote/decentralized trials (DCTs) – with ePRO, eConsent, and telemedicine integrations.
EDC is suited to any trial where electronic data capture, validation, and monitoring are needed. It reduces errors, speeds up access to data, and increases regulatory compliance.
What is the difference between EDC and eCRF?
The terms are closely related but refer to two different facets of the data collection process:
EDC (Electronic Data Capture) refers to the overall software system utilized to capture, store, and manage clinical trial data electronically. It includes various tools and features like validation checks, audit trails, query management, and data export.
eCRF (electronic Case Report Form) is the electronic equivalent in the EDC system where site staff enter patient information. An eCRF is a specific visit or procedure and is generated as per the study protocol.
What factors should you consider when choosing an EDC system?
The right choice of EDC system is critical to the success of your clinical trial. Among the most significant considerations to keep in mind are:
- Ease of use: The system should be easy to use for both site personnel and sponsors, minimizing the need for extensive training.
- Configurability: Easily configurable form design, workflow, and edit checks with minimal coding to your study protocol are crucial.
- Compliance and security: Ensure the system meets industry standards like GCP, 21 CFR Part 11, and GDPR, and offers secure data storage with audit trails and role-based access.
- Integration capabilities: Consider if the EDC can be integrated with your CTMS, eTMF, ePRO, or external laboratory systems via API.
- Support and validation: Good vendor support, system validation documentation, and responsiveness are paramount especially during study setup and live phases.
- Cost and scalability: Evaluate licensing models, set-up fees, and whether the system will scale with your study needs – from small studies to big, multi-site, global trials.
- Real-time access and reporting: Look for dashboards, export features, and monitoring tools that give stakeholders real-time visibility into study progress.
What are the usual pitfalls in transitioning from paper to EDC systems?
Transitioning from paper to electronic data capture provides many benefits but with some issues also that organizations need to be prepared to face:
- Change management: Paper-based teams may resist the change. Training and proper communication are essential to establish user confidence and acceptance.
- Initial setup and configuration: Development of electronic Case Report Forms (eCRFs), validation rules, and business processes requires time and effort from clinical and data management personnel.
- Training and user acceptance: Site personnel, monitors, and data managers must be trained on the new system, which may temporarily decrease study operations if poorly supported.
- Technical infrastructure: Consistent internet connectivity, appropriate devices, and secure environments must be provided at every site that is part of the study, some of which may need to be upgraded.
- Cost implications: While EDC can save money in the long term, initial expenses for licensing, training, and implementation can be daunting to smaller organizations or studies.
- Regulatory compliance and validation: Having the system in regulatory compliance (e.g., 21 CFR Part 11) and validated is essential and can be challenging for first-time users.
What impact do EDC systems have on the overall cost of clinical trials?
EDC systems can significantly contribute to reducing the overall cost of clinical trials, especially in later stages. Even though there is an upfront cost of installation, licensing, and training, these systems yield cost savings in the long term by:
- Reducing data entry errors: fewer errors mean less time invested in query resolution and data cleansing
- Condensing timelines: access to live data speeds up decision-making, monitoring, and reporting
- Decreasing paper and storage costs: no need to store physical CRFs, no manual filing
- Decreasing monitoring expenses: access to remote data reduces the number of site visits required
- Increasing efficiency: automated processes and integrations decrease manual labor between teams
In short, EDC systems help to streamline processes and remove costs buried in paper-based processes ultimately maximizing return on investment to the trial.



