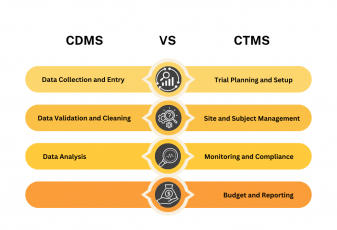
Clinical Trials are notoriously expensive to operate, with the majority of trial sites working with no more than a 3-month operating budget. That means that maintaining up-to-date financial records, and responsibly using what funds do exist is more essential than ever before. With an estimated two-thirds of trial sites falling into this category, software companies have sought to close this gap in effective financial operations with process automation and payment scheduling by designing software suites that address the problems faced by the companies who operate and manage clinical trials.
Likewise, effective subject management is essential to producing meaningful results. Sometimes this means coordinating across several physical locations, and other times it means the organization of large groups of people. No matter what the problem is though, effective solutions mean the realization and production of a permanent solution that balances the robust functionality of a complex network and the usability of a program that may be accessed daily.
What are the problems exactly though?
- Disorganized payments systems that are the result of managing financial inflows and outflows across too wide a spectrum.
- The inability to scale payment practices to meet trial requirements.
- Challenges in tracking subjects and advancing study protocol.
With the Subject Tracking & Invoicing module from Flex Databases, essential problems may be solved and secondary may be resolved as well with a range of tools that are provided in even the baseline version of the module.
The Flex Databases Application Environment is specifically designed for the companies that we serve. Our system manages and automates a number of essential processes so that payments are distributed on time and properly invoiced.